A solution is believed to contain one or more of the following ions: Cr 3+ , Zn
Question:
A solution is believed to contain one or more of the following ions: Cr3+, Zn2+, Fe3+, Ni2+. When the solution is treated with excess NaOH(aq), a precipitate forms. The solution in contact with the precipitate is colorless. The precipitate is dissolved in HCl(aq), and the resulting solution is treated with NH3(aq). No precipitation occurs. Based solely on these observations, what conclusions can you draw about the ions present in the original solution? That is, which ion(s) are likely present, which are most likely not present, and about which can we not be certain? Refer to Appendix D for solubility product and complex-ion formation data.
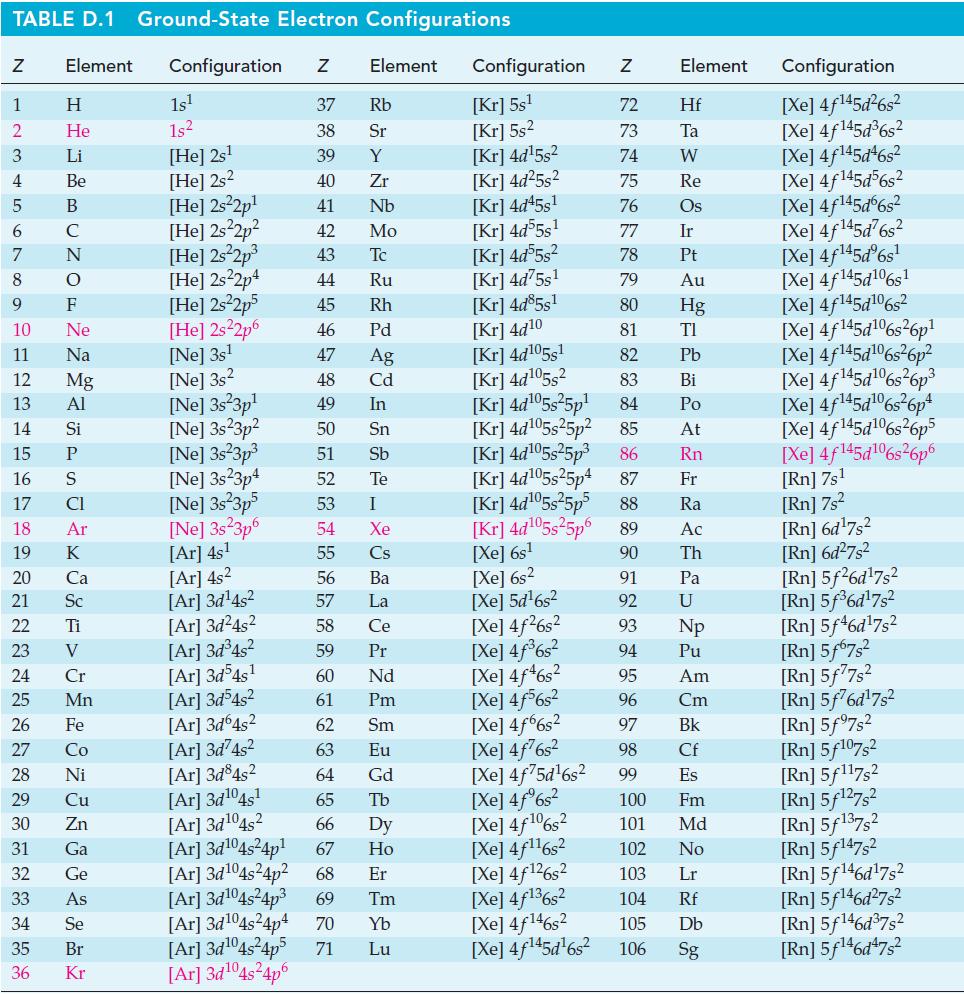
TABLE D.1 Ground-State Electron Configurations Element Configuration Z Z 1 2 3 4 6 7 8 9 5 B 10 11 12 13 14 15 16 17 18 19 20 25 27 29 HIGÅMUZONSUZ SE> 0 ≤ 2 3 2 3 5 3 3 4 8 5 2 30 Η 31 He 32 Li 33 Be C F Ne Na Mg Al 21 Sc 22 Ti 23 V 24 Si P CI Ar 26 Fe K Ca 28 Ni Cr Mn Co Cu Zn Ga Ge As 34 Se 35 Br 36 Kr 1s¹ 1s² [He] 2s¹ [He] 2s2 [He] 2s²2p¹ [He] 2s²2p² [He] 2s²2p³ [He] 2s22p4 [He] 2s²2p5 [He] 2s²2p6 [Ne] 3s¹ [Ne] 3s2 [Ne] 3s 3p¹ [Ne] 3s23p² 37 Rb 38 Sr 39 Y 40 Zr 41 Nb 42 Mo 43 Tc 44 Ru 45 Rh 46 Pd 47 Ag 48 Cd 49 In 50 Sn 51 Sb Te 52 53 I 54 Xe 55 Cs 56 Ba 57 La 58 Ce 59 Pr [Ar] 3d³4s² [Ar] 3d54s¹ 60 Nd 61 Pm 62 Sm 63 Eu [Ar] 3d³4s² [Ar]3d64s² [Ar]3d²4s² [Ar]3d845² [Ar] 3d¹04s¹ [Ar] 3d¹04s2 64 Gd 65 Tb 66 67 [Ar] 3d¹04s²4p¹ Dy Ho Er 69 Tm [Ar] 3d¹04s²4p² 68 [Ar]3d¹04s²4p³ [Ar] 3d¹04s²4p4 70 Yb Lu [Ne] 3s²3p³ [Ne] 3s23p4 [Ne] 3s 3p5 [Ne] 3s 3p6 [Ar] 4s¹ [Ar] 4s² [Ar]3d¹4s² [Ar]3d²4s² [Ar]3d¹04s²4p5 71 Element [Ar]3d¹04s²4p6 Configuration Z [Kr] 5s¹ [Kr] 5s² [Kr] 4d¹5s² [Kr] 4d²5s² [Kr] 4d45s¹ [Kr] 4d55s¹ [kr] 4d55s² [Kr] 4d75s¹ [Kr] 4d85s1 [Kr] 4d10 [Kr] 4d105s1 [Kr] 4d¹05s² [Kr] 4d¹05s²5p¹ [Kr] 4d¹05s25p² [Kr] 4d¹05s²5p³ [kr] 4d¹05s25p4 [Xe] 6s² [Xe] 5d¹6s² [Xe] 4f²6s² [Xe] 4f³6s² [Xe] 4f46s2 [Xe] 4f6s2 [Xe] 4f6s2 [Xe] 4f76s2 [Xe] 4f75d¹6s² [Xe] 4f%s2 [Xe] 4f106s2 [Xe] 4f¹¹6s² NRNKERKR [Xe] 4f126s2 [Xe] 4f136s2 [Xe] 4f146s2 [Xe] 4f¹45d¹6s² 72 Hf 73 Ta W 74 75 Re 76 Os 77 Element 78 79 80 81 82 83 84 85 86 87 Fr [Kr] 4d¹05s²5p5 88 Ra [Kr] 4d¹05s²5p6 89 Ac [Xe] 6s¹ 90 Th 91 92 93 94 95 96 97 98 99 Ir Pt Au Hg TI Pb Bi Po At Rn Pa U Np Pu Am Cm Bk Cf Es 100 Fm 101 Md 102 No 103 Lr 104 Rf 105 Db 106 Sg Configuration [Xe] 4f¹45d²6s² [Xe] 4f145d³6s² [Xe] 4f145d46s2 [Xe] 4f145d56s2 [Xe] 4f145d6s2 [Xe] 4f¹45d²6s² [Xe] 4f¹45dº6s¹ [Xe] 4f145d106s1 [Xe] 4f145d106s2 [Xe] 4f145d6s26p* [Xe] 4f145d106s36p? [Xe] 4f145d16s?6p3 [Xe] 4f145d6s®6p* [Xe] 4f145d16s26p5 [Xe] 4f145d106s26p6 [Rn] 7s¹ [Rn] 7s² [Rn] 6d¹7s² [Rn] 6d²7s² [Rn] 5f26d¹7s² [Rn] 5f³6d¹7s² [Rn] 5f46d¹7s2 [Rn] 5f67s² [Rn] 5f77s² [Rn] 5f76d¹7s² [Rn] 5f97s2 [Rn] 5f107,2 [Rn] 5f117s2 [Rn] 5f¹27s² [Rn] 5f137,2 [Rn] 5f147s2 Rn] 5f¹46d¹7s² [Rn] 5f¹46d²7s² [Rn] 5f¹46d³7s2 [Rn] 5f¹46d¹7s²
Step by Step Answer:

This question has not been answered yet.
You can Ask your question!

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Students also viewed these Sciences questions
-
Managing Scope Changes Case Study Scope changes on a project can occur regardless of how well the project is planned or executed. Scope changes can be the result of something that was omitted during...
-
THIRD AVENUE SOFTWARE HEALTH-CARE APP PROJECT This case is new for the ninth edition of Information Technology Project Management . The case provides an opportunity to apply agile and Scrum...
-
[A] Aon Corporations Mezzanine Preferred Stock In its 2002 annual report to shareholders, Aon Corporation described its mandatorily redeemable preferred stock as follows: In January 1997, Aon created...
-
Which of the following statements best describes the 'dilation' in rock mechanics? a. Movement of the rock along the shear direction during the direct shear tests b. Radial expansion of the intact...
-
Jason Company needs to estimate the inventory balance for its quarterly financial statements. The periodic inventory method is used. Records show that quarterly sales totaled $550,000, beginning...
-
Janice Morgan, age 24, is single and has no dependents. She is a freelance writer. In January 2015, Janice opened her own office located at 2751 Waldham Road, Pleasant Hill, NM 88135. She called her...
-
Surviving Surgery A doctor gives a patient a 60% chance of surviving bypass surgery after a heart attack. If the patient survives the surgery, he has a 50% chance that the heart damage will heal....
-
Children's Hospital of the King's Daughter in Norfolk, Virginia, introduced a new budgeting method that allowed the hospital's annual plan to be updated for changes in operating plans. For example,...
-
The following information is for the Vista Company for the year; the company sells just one product: Units Unit Cost Beginning Inventory Jan. 1 200 $10 Purchases: Feb. 11 500 14 May 18 400 17 Oct. 23...
-
A 0.960 g sample of impure hematite (Fe 2 O 3 ) is treated with 1.752 g of oxalic acid (H 2 C 2 O 4 2 H 2 O) in an acidic medium. Following this, the excess oxalic acid is titrated with 35.16 mL of...
-
Show that under the following conditions, Ba 2+ (aq) can be separated from Sr 2+ (aq) and Ca 2+ (aq) by precipitating BaCrO 4 (s) with the other ions remaining in solution: Use data from this and...
-
The following are selected account balances of Rule Corporation at the end of 2016: Rule is subject to a 30% income tax rate, and shareholders own 800 shares of its capital stock. Required: Prepare a...
-
The financial statements for the Columbia Sportswear Company can be found in Appendix A, and Under Armour, Inc.'s financial statements can be found in Appendix B at the end of this book. Required a....
-
Use the data from SE3-8 to prepare the closing entries for The Decade Company. Close the temporary accounts to income summary. The balance of \(\$ 8,500\) in the retained earnings account is from the...
-
Adjusting Entries The following selected accounts appear in the Birch Company's unadjusted trial balance as of December 31, the end of the fiscal year (all accounts have normal balances): Required...
-
Closing Entries Use the information provided in E3-5A to prepare journal entries to close the accounts using the Income Summary account. After these entries are posted, what is the balance in the...
-
Ceva, Inc. manufactures and services jet engines for air carriers. The engines cost \($10\) to \($40\) million each, depending on the specifications and plane. A 10-year service contract for a single...
-
(a) Construct a decoding table (with syndromes) for the group code given by the generator matrix (b) Use the table from part (a) to decode the following received words. 11110 11101 11011 10100 10011...
-
Research an article from an online source, such as The Economist, Wall Street Journal, Journal of Economic Perspectives, American Journal of Agricultural Economics, or another academic journal. The...
-
Delmont Company entered into these transactions during May 2012. 1. Purchased computers for office use for $30,000 from Dell on account. 2. Paid $4,000 cash for May rent on storage space. 3. Received...
-
During 2012, its first year of operations as a delivery service, Underwood Corp. entered into the following transactions. 1. Issued shares of common stock to investors in exchange for $100,000 in...
-
A tabular analysis of the transactions made during August 2012 by Nigel Company during its first month of operations is shown below. Each increase and decrease in stockholders?? equity is explained....
-
3. The nominal interest rate compounded monthly when your $7,000 becomes $11,700 in eight years is ________
-
An investor can design a risky portfolio based on two stocks, A and B. Stock A has an expected return of 21% and a standard deviation of return of 39%. Stock B has an expected return of 14% and a...
-
Advanced Small Business Certifica Drag and Drop the highlighted items into the correct boxes depending on whether they increase or decrease Alex's stock basis. Note your answers- you'll need them for...

Study smarter with the SolutionInn App


