A 0.960 g sample of impure hematite (Fe 2 O 3 ) is treated with 1.752 g
Question:
A 0.960 g sample of impure hematite (Fe2O3) is treated with 1.752 g of oxalic acid (H2C2O4 · 2 H2O) in an acidic medium. Following this, the excess oxalic acid is titrated with 35.16 mL of 0.100 M KMnO4. What is the mass percent of Fe2O3 in the impure sample of hematite? The following equations are neither complete nor balanced.
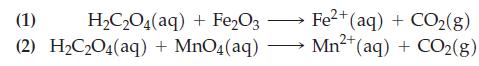
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:





