Acetylene (C 2 H 2 ) torches are used in welding. How much heat (in kJ) evolves
Question:
Acetylene (C2H2) torches are used in welding. How much heat (in kJ) evolves when 5.0 L of C2H2 (d = 1.0967 kg/m3) is mixed with a stoichiometric amount of oxygen gas? The combustion reaction is
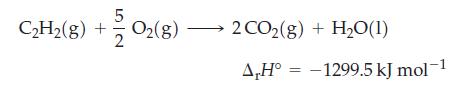
Transcribed Image Text:
5 C₂H₂(g) + +2/202(8) 2 CO2(g) + H₂O(1) A,H° -1299.5 kJ mol-1
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
First lets balance the chemical equation to make sure we know how many moles of reactants and pr...View the full answer

Answered By

Sandhya Sharma
I hold M.Sc and M.Phil degrees in mathematics from CCS University, India and also have a MS degree in information management from Asian institute of technology, Bangkok, Thailand. I have worked at a international school in Bangkok as a IT teacher. Presently, I am working from home as a online Math/Statistics tutor. I have more than 10 years of online tutoring experience. My students have always excelled in their studies.
4.90+
119+ Reviews
214+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
Nitric acid is used extensively for the production of inorganic and organic nitrates, for metal treatments of various kinds, and for photoengraving. It is produced by oxidizing ammonia to nitric...
-
A flow of hydrogen gas is mixed with a flow of oxygen in a stoichiometric ratio, both at 298 K and 50 kPa. The mixture burns without any heat transfer in complete combustion. Find the adiabatic flame...
-
A Claus plant converts gaseous sulfur compounds to elemental sulfur, thereby eliminating emission of sulfur into the atmosphere. The process can be especially important in the gasification of coal,...
-
A random sample of 100 students was taken from a large university to study the relationship between GPA and the number of hours of study per week. The following linear regression equation was...
-
Three inventory categories are reported on a manufacturing companys balance sheet: (a) Raw materials, (b) Goods in process, and (c) Finished goods. Identify the usual order in which these inventory...
-
Create an example of an entity with an optional attribute.
-
WACC The Patrick Companys year-end balance sheet is shown below. Its cost of common equity is 16%, its before-tax cost of debt is 13%, and its marginal tax rate is 40%. Assume that the firms...
-
Winter Garden is a luxury hotel with 150 suites. Its regular suite rate is $250 per night per suite. The hotels cost per night is $140 per suite and consists of the following. Variable direct labor...
-
Please show details, Give Kudos! DUESTION 3: Percentage of Completion Method Lilia Construction Company has contracted to build an office building. The construction began on January 1, 2020, and the...
-
Lu Ltd. has experienced the following accounting earnings and taxable income: The differences between accounting and taxable income are caused by differences between accounting and tax expenses that...
-
The heat of neutralization of HCl(aq) by NaOH(aq) is -55.84 kJ/mol H 2 O produced. If 50.00 mL of 1.05 M NaOH is added to 25.00 mL of 1.86 M HCl, with both solutions originally at 24.72 C, what will...
-
Refer to Example 7-4. The product of the neutralization is 0.500 M NaCl. For this solution, assume a density of 1.02 g/mL and a specific heat capacity of 4.02 J g -1 C -1 . Also, assume a heat...
-
Look at Table 12.1 and Figure 12.7 in the text. When were T-bill rates at their highest over the period from 1926 through 2019? Why do you think they were so high during this period? What...
-
The Capital Asset Pricing Model (CAPM) says that the risk premium on security \(j\) is related to the risk premium on the market portfolio, that is where \(r_{j}\) and \(r_{f}\) are the returns to...
-
Verify the likelihood (8.16). n t-1 II (Pt, (xi) i (1 Pt, (x;))(1-cs) II (1 Pk (xi)) - (8.16) i=1 k=1
-
Check that [edgm \(][\) cdgm \(]\) is a graphical model. Education Gender MS Dep CIRS
-
Use the information provided in P3-9B. Required a. Prepare closing entries at December 31 in general journal form using the Income Summary account. b. After the closing entries are posted, calculate...
-
If the light bulb in Figure \(33.8 a\) is \(1.0 \mathrm{~m}\) in front of the mirror, how far behind the mirror is the image? Data from Figure 33.8a (a) Rays shows path of light that travels from...
-
Describe some of the ways in which a metal matrix can be introduced into a fiber-reinforced composite.
-
For the following exercises, rewrite the sum as a product of two functions or the product as a sum of two functions. Give your answer in terms of sines and cosines. Then evaluate the final answer...
-
Installment Repossession Entries selected transactions of TV Land Company are presented below. 1. A television set costing $540 is sold to Jack Matre on November 1, 2010, for $900. Matre makes a down...
-
Installment-Sales Computations and Schedules Saprano Company, on January 2, 2010, entered into a contract with a manufacturing company to purchase room-size air conditioners and to sell the units on...
-
Completed-Contract Method) Monat Construction Company, Inc., entered into a firm fixed price contract with Hyatt Clinic on July 1, 2010, to construct a four-story office building. At that time, Monat...
-
Stockholders Equity Transactions, Journal Entries, and T-Accounts The stockholders equity of Fremantle Corporation at January 1 follows: 8 Percent preferred stock, $110 par value, 20,000 shares...
-
How does tax evasion differ from tax avoidance?
-
Question 14 (1 point) Android is used as a non-mobile operating system. True False

Study smarter with the SolutionInn App


