Assume that all reactants and products are in their standard states, and use data from Table 19.1
Question:
Assume that all reactants and products are in their standard states, and use data from Table 19.1 to predict whether a spontaneous reaction will occur in the forward direction in each case.
Table 19.1
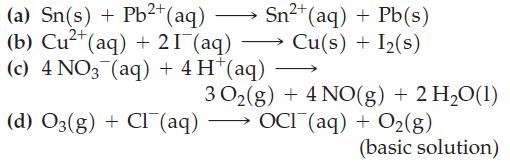
Transcribed Image Text:
(a) Sn(s) + Pb²+ (aq) (b) Cu²+ (aq) + 21 (aq) (c) 4 NO3 (aq) + 4 H*(aq) (d) O3(g) + Cl(aq) Sn²+ (aq) + Pb(s) Cu(s) + I₂(s) 30₂(g) + 4 NO(g) + 2 H₂O(1) OCI (aq) + O₂(g) (basic solution)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (QA)
To determine whether a reaction is spontaneous in the forward direction we can use standard reductio...View the full answer

Answered By

Munir Ahmed Jakhro
I am professional Tutor of of Business Courses, I did my four years Bachelor Degree from one of the Top Business schools of World "Institute of Business Administration" in year 2013. Since then I have been working as Tutor of Accounting, Finance tutor on different online platforms like this website. I am have experience of 6 years teaching business courses to students online and offline my professional job at national savings also helped me in accounting understanding .
4.90+
8+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
Which of the following reactions occur spontaneously, and which can be brought about only through electrolysis, assuming that all reactants and products are in their standard states? For those...
-
Write an equation to represent the oxidation of Cl - (aq) to Cl 2 (g) by PbO 2 (s) in an acidic solution. Will this reaction occur spontaneously in the forward direction if all other reactants and...
-
According to Le Chteliers principle and the dependence of free energy on reactant and product concentrations, which statement is true? (Assume that both reactants and products are gaseous.) (a) A...
-
Two stocks, A and B, have beta coefficients of 0.8 and 1.4, respectively. If the expected return on the market is 10 percent and the risk-free rate is 5 percent, what is the risk premium associated...
-
Using the following information, compute cash flow from operating activities under (1) the U.S. approach and (2) the U.K. approach. (a) Cash paid to purchase inventory . . . . . . . . . . . . . . . ....
-
How does average height for boys change as boys get older? According to Physicians Handbook, the average heights at different ages are: Make a time-series graph for average height for ages 0.514...
-
Domestic seafood processing. Each year, the National Oceanic Atmospheric Association (NOAA) publishes a report titled Fisheries of the United States. One part of the report gives annual data on...
-
You are analyzing various programs to reduce water pollution from food processing plants. In consultation with your staff, you have developed the following matrix of effects (where PV = Present...
-
Pharoah Industries has a three-year bond outstanding that pays a 7.35 percent coupon rate and is currently priced at $938.15. What is the yield to maturity of this bond? Assume annual coupon...
-
Ni 2+ has a more positive reduction potential than Cd 2+ . (a) Which ion is more easily reduced to the metal? (b) Which metal, Ni or Cd, is more easily oxidized?
-
For the reduction half-cell reactions Hg 2 2+ (aq) + 2 e 2 Hg(l), E = 0.797 V. Will Hg(l) react with and dissolve in HCl(aq)? in HNO 3 (aq)? Explain.
-
Step 1 Decide on some timed event that you can regularly sample, either by yourself or (preferably) as a group. This could be the arrival time of your colleagues at work each morning, the start time...
-
Charlotte, a marketing manager, is worried her firm is doing a poor job of managing the movement of finished products to the final consumer. If she is right, the company should work to improve its Mul
-
Sketch a graph of the piecewise defined function. f(x) = Sx if x 0 x+9 if x>0
-
Complete the information for the following subnetting problem. Your answers should be whole numbers without a period. Example: the correct value for the last octet of 172.20.55.210 would be entered...
-
Olympia Trophy Company wants to purchase a laser engraving machine to use in its production of trophies. The cost of this engraving machine is $69,546.40 and it will yield yearly expected cash flows...
-
Scenario: Envirotruck is a company that produces energy efficient all-wheel-drive and 4-wheel-drive trucks (twice as efficient as their competition's) with ample clearance for construction and rough...
-
Plot the final bend radius as a function of initial bend radius in bending for (a) 5052-O aluminum, (b) 5052-H34 aluminum, (c) C24000 brass, (d) AISI 304 stainless steel sheet.
-
Determine whether the lines are parallel, perpendicular, or neither. 2x + 3y = -12, 2y - 3x = 8
-
Blanco Metal Company produces the steel wire that goes into the production of paper clips. In 2010, the first year of operations, Blanco produced 50,000 miles of wire and sold 45,000 miles. In 2011,...
-
Gagliano Company has decided to introduce a new product. The new product can be manufactured by either a capital-intensive method or a labor-intensive method. The manufacturing method will not affect...
-
The condensed income statement for the Terri and Jerri partnership for 2010 is as follows. A cost behavior analysis indicates that 75% of the cost of goods sold are variable, 50% of the selling...
-
Fig 1. Rolling a 4 on a D4 A four sided die (D4), shaped like a pyramid (or tetrahedron), has 4 flat surfaces opposite four corner points. A number (1, 2, 3, or 4) appears close to the edge of each...
-
I just need help with question #4 please! Thank you! Windsor Manufacturing uses MRP to schedule its production. Below is the Bill of Material (BOM) for Product A. The quantity needed of the part...
-
(25) Suppose that we have an economy consisting of two farmers, Cornelius and Wheaton, who unsurprisingly farm corn c and wheat w, respectively. Assume that both farmers produce their crop of choice...

Study smarter with the SolutionInn App


