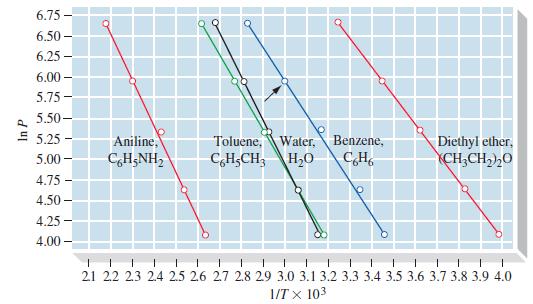
By the method used to graph Figure 12-20, plot ln P versus 1/T for liquid white phosphorus,
Question:
By the method used to graph Figure 12-20, plot ln P versus 1/T for liquid white phosphorus, and estimate
(a) Its normal boiling point and
(b) Its enthalpy of vaporization, ΔvapH, in kJ mol-1. Vapor pressure data: 76.6 °C, 1 mmHg; 128.0 °C, 10 mmHg; 166.7 °C, 40 mmHg; 197.3 °C, 100 mmHg; 251.0 °C, 400 mmHg.
Figure 12-20
Transcribed Image Text:
6.75- 6.50- 6.25 6.00- 5.75- 5.50- E 5.25- 5.00- 4.75 4.50- 4.25- 4.00- - Q Aniline, CHẠNH, 09 Toluene, Water, C6H-CH3 H₂O Q Benzene, CH Diethyl ether, CH3CH₂)20 1 1 1 1 1 1 I 1 1 1 1 1 T 1 21 22 23 2.4 2.5 2.6 2.7 2.8 2.9 3.0 3.1 3.2 3.3 3.4 3.5 3.6 3.7 3.8 3.9 4.0 1/TX 103
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 33% (3 reviews)
To plot ln P versus 1T for liquid white phosphorus we first ...View the full answer

Answered By

Joseph Mwaura
I have been teaching college students in various subjects for 9 years now. Besides, I have been tutoring online with several tutoring companies from 2010 to date. The 9 years of experience as a tutor has enabled me to develop multiple tutoring skills and see thousands of students excel in their education and in life after school which gives me much pleasure. I have assisted students in essay writing and in doing academic research and this has helped me be well versed with the various writing styles such as APA, MLA, Chicago/ Turabian, Harvard. I am always ready to handle work at any hour and in any way as students specify. In my tutoring journey, excellence has always been my guiding standard.
4.00+
1+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
Liquid nitrogen is stored in 0.5-m3 metal tanks that are thoroughly insulated. Consider the process of filling an evacuated tank, initially at 295 K. It is attached to a line containing liquid...
-
You are going to prepare a silicone polymer, and one of the starting materials is dichlorodimethylsilane, SiCl 2 (CH 3 ) 2 . You need its normal boiling point and to measure equilibrium vapor...
-
A horizontal spring is attached to the wall. The spring has a force constant k =1980 N/m and is compressed 0.08 m from its normal length. A block of mass 0.2kg is placed a rest against the spring....
-
The fieldwork for the 30 June 20X0 audit of Tracy Brewing Company Ltd was finished on 19 August 20X0 and the completed financial statements, accompanied by the signed audit reports, were mailed on 6...
-
Holly Hill Acres, Ltd. executed and delivered a promissory note and a purchase money mortgage to Rogers and Blythe. The note provided that it was secured by a mortgage on certain real estate and that...
-
When might reliance form the basis for contract rights and duties despite a lack of consideration?
-
Do you reflect upon an inner life of people and yourself?
-
1. Why does Rorty suggest that the discussion of relativism is too important to be left to philosophy professors? 2. What does Rorty mean when he says that the Kantian or Enlightenment focus on...
-
21. Juenemann Kennel uses tenant-days as its measure of activity, an animal housed in the kennel for one day is counted as one tenant-day. During December , the Kennel budgeted for 3.800 tenant-days,...
-
Complete Form 941 for the 4th quarter for TCLH Industries (which is located at 202 Whitmore Avenue, Durham, NC 27701; Employer Identification #44-4444444). Assume that all necessary deposits were...
-
One handbook lists the sublimation pressure of solid benzene as a function of Kelvin temperature, T, as log P (mmHg) = 9.846 - 2309/T. Another handbook lists the vapor pressure of liquid benzene as a...
-
Because solid p-dichlorobenzene, C 6 H 4 Cl 2 sublimes rather easily, it has been used as a moth repellent. From the data given, estimate the sublimation pressure of C 6 H 4 Cl 2 (s) at 25 C For C 6...
-
Your firm has a new individual client, Carla Navarro, who has been assigned to you for preparation of the current year's tax return. Upon review of Carla's tax returns from prior years, you notice...
-
A light, inextensible cord passes over a frictionless pulley as shown in figure below. One end of the rope is attached to a block, and a force P is applied to the other end. Block A weighs 600 lb and...
-
BASICOT POST DO NOT ASSIST DO NOT POST DO NOT ASSIST DO NOT POST DO NOT ASSIST For filming a physics demonstration about oscillation, an educational video crew attaches a large spring to a very small...
-
Day Mail Order Co. applied the high-low method of cost estimation to customer order data for the first 4 months of the year. What is the estimated variable order-filling cost component per order...
-
The four forces, 400, 500, 600 and 700N are acting along the edges of a 0.8m cube as shown. Represent the resultant of these forces by 1) A force Fr through the point A 2) A couple moment Mr (give...
-
Problem 1. What is the degree of freedom of the following mechanism? Sliding joint Sliding joint
-
How many peaks will there be in the decoupled 13C NMR spectrum of each of the following compounds? CH3 NH, c. b. CH3CH2-C-OH CH3 a.
-
If a test has high reliability. O the test measures what the authors of the test claim it measures O people who take the same test twice get approximately the same scores both times O scores on the...
-
Call Provisions TMCC has the right to buy back the securities on the anniversary date at a price established when the securities were issued (this feature is a term of this particular deal). What...
-
Time Value of Money would you be willing to pay $1.163 today in exchange for $10,000 in 30 years? What would be the key considerations in answering yes to no? Would your answer depend on who is...
-
Investment Comparison suppose that when TMCC offered the security for $1,163 the U.S. Treasury had offered an essentially identical security. Do you think it would have had a higher or lower price?...
-
(Future value)If you deposit $2,300 today into an account earning an annual rate of return of 11 percent, what would your account be worth in 20 years (assuming no further deposits)? In 25 years?...
-
You receive a cash dividend of $700 that is fully franked (i.e. has the maximum amount of franking credits attached). In relation to this dividend, the total amount that you must include in your...
-
Suppose a smoker indicates on an application for medical expense insurance that he is a nonsmoker. Is this misrepresented material?

Study smarter with the SolutionInn App


