Calculate the aqueous solubility, in moles per liter, of each of the following. (a) BaCrO4, Ksp =
Question:
Calculate the aqueous solubility, in moles per liter, of each of the following.
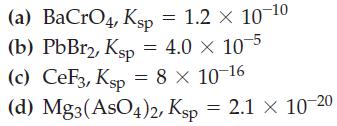
Transcribed Image Text:
(a) BaCrO4, Ksp = 1.2 x 10-10 (b) PbBr2, Ksp = 4.0 × 10-5 (c) CeF3, Ksp = 8 × 10-16 (d) Mg3(AsO4)2, Ksp = 2.1 × 10-20
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (4 reviews)
The solubility product constant Ksp describes the equilibrium between the dissolved ions and the solid compound at saturation It can be used to calcul...View the full answer

Answered By

OTIENO OBADO
I have a vast experience in teaching, mentoring and tutoring. I handle student concerns diligently and my academic background is undeniably aesthetic
4.30+
3+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
Calculate the solubility of each of the following compounds in moles per liter and grams per liter. (Ignore any acidbase properties.) a. Ag3PO4, Ksp = 1.8 10-18 b. CaCO3, Ksp = 8.7 10-9
-
What is the solubility in moles per liter of Ca(OH)2? Use data from Table 13.2.
-
What is the solubility in moles per liter of AgCl? Use data from Table 13.2.
-
Sandys Socks makes the worlds best socks. Information for the last eight months follows: Prepare a scatter graph by plotting Sandys data on a graph. Then draw a line that you believe best fits the...
-
Below are balance sheet and income statement data for Blue Panel Corporation. Additional information for Blue Panel Corporation is as follows: (a) Property, plant, and equipment with an original...
-
A $10 000, 10% bond with quarterly coupons redeemable at par in 15 years is purchased to yield 11% compounded quarterly. Determine the purchase price of the bond.
-
The table below gives the prices for three products (A, B, and C) for the four quarters of last year. Quarter A B C 1 3.25 1.75 8.00 2 3.50 1.25 9.35 3 3.90 1.20 9.70 4 4.25 1.00 10.50 a. Compute a...
-
Five months before the new 2002 Lexus ES hit showroom floors, the company's U.S. engineers sent a test report to Toyota City in Japan: The luxury sedan shifted gears so roughly that it was "not...
-
5 . Mark Mower paid Invoice Number 6 8 0 on March 2 6 . Enter the receipt on accountin the proper journal.Note: Be sure to record the Sales Discount . 6 . Record the following employees salaries in...
-
Arrange the following solutes in order of increasing molar solubility in water: AgCN, AgIO 3 , AgI, AgNO 2 , Ag 2 SO 4 . Explain your reasoning.
-
The following K sp values are found in a handbook. Write the solubility product expression to which each one applies. For example, K sp( AgCl) = [Ag + ][Cl] = 1.8 x 10 -10 . (a) Kep(CrF3) = 6.6 10-1...
-
What is the marginal rate of return? How is it calculated?
-
Avery, an unmarried taxpayer, had the following income items: Salary Net income from a rental house 3 7 , 0 5 0 4 , 9 0 0 Avery has a 4 - year - old child who attends a child care center. Assume the...
-
California Lottery Let A denote the event of placing a $1 straight bet on the California Daily 4 lottery and winning. There are 10,000 different ways that you can select the four digits (with...
-
"Tamara Wiley glanced in the mirror before leaving her apartment and heading to her 8 a.m. class. She was having a bad hair day, so she had thrown on a scarf. Her quick check in the mirror told her...
-
Online Friends In a Pew Research Center survey of 1060 teens aged 13 to 17, it was found that 604 (or 57.0%) of those respondents have made new friends online. If the true rate is 50%, there is a...
-
Dr. Yong has requested that Senture Houston, an office manager at Pain Free Dental Associates, prepare a single journal entry for December 31, 2022. The bank statement for that day shows $9,500....
-
Give examples of (a) Forced vibration (b) Self-excited vibration in general engineering practice.
-
You've been asked to take over leadership of a group of paralegals that once had a reputation for being a tight-knit, supportive team, but you quickly figure out that this team is in danger of...
-
(a) What is the major rationale for the use of variable costing? (b) Discuss why variable costing may not be used for financial reporting purposes.
-
Monthly production costs in Pesavento Company for two levels of production are as follows. Indicate which costs are variable, fixed, and mixed, and give the reason for eachanswer. 3,000 units 6,000...
-
For Loder Company, the relevant range of production is 4080% of capacity. At 40% of capacity, a variable cost is $4,000 and a fixed cost is $6,000. Diagram the behavior of each cost within the...
-
How to solve general ledger cash balance chapter 9 assignment 5
-
On 31 July 2018, Sipho bought 1 000 ordinary shares in ABC Ltd at a cost of R2 750. On 31 December 2018 the company made a 1 for 10 bonus issue. On 31 March 2019, Sipho sold 300 shares for R800. What...
-
If you purchase a $1000 par value bond for $1065 that has a 6 3/8% coupon rate and 15 years until maturity, what will be your annual return? 5.5% 5.9% 5.7% 6.1%

Study smarter with the SolutionInn App


