Calculate the entropy change, S, for the following processes. If necessary, look up required data in Appendix
Question:
Calculate the entropy change, ΔS, for the following processes. If necessary, look up required data in Appendix D.
(a) A mole of He(g) undergoes an expansion from V to 2V at 298 K.
(b) The temperature of one mole of CH4(g) is increased from 298 K to 325 K at a constant pressure of 1 bar.
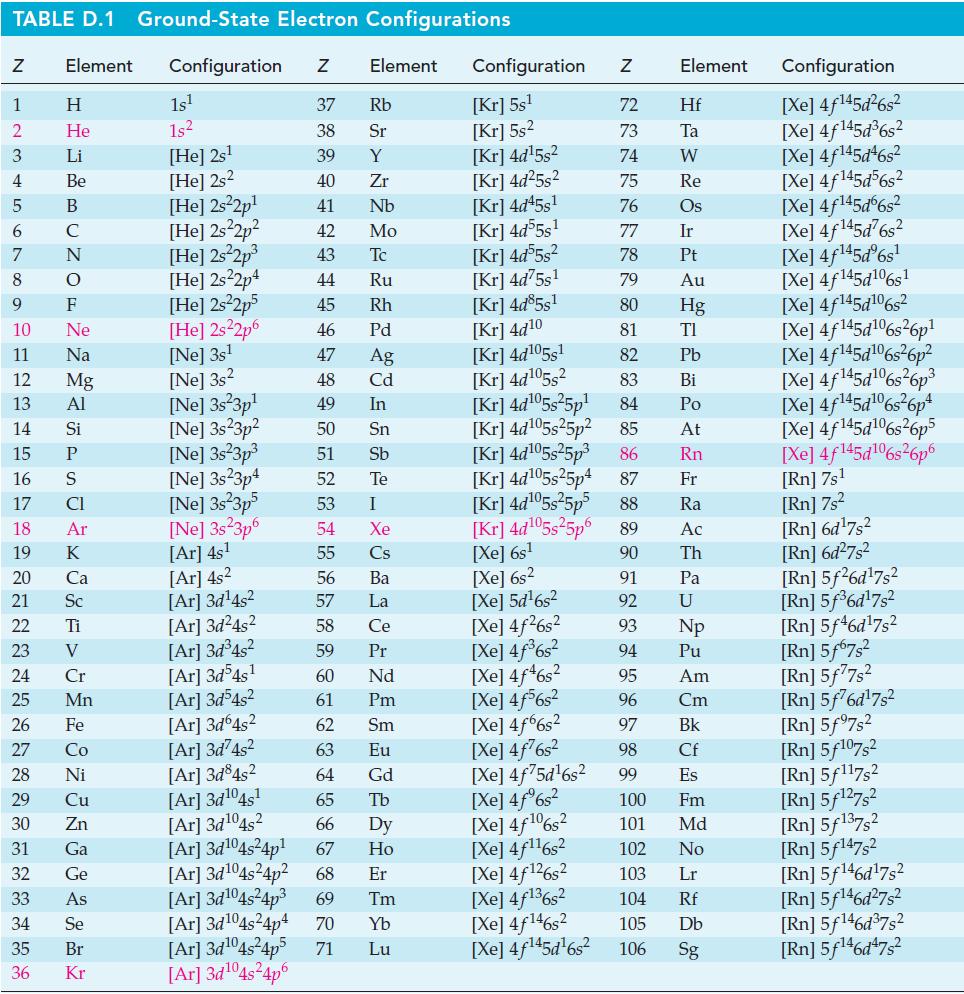
Transcribed Image Text:
TABLE D.1 Ground-State Electron Configurations Element Configuration Z Z 1 2 3 4 5 6 7 8 9 10 11 12 13 14 16 17 18 19 24 25 HIGÅ LUZONS JY E> 0 ≤ 2 3 2 3 5 3 3 2 2 5 2 27 Η 15 P He 29 Li 30 Be 31 B 32 C 33 F Ne Na 20 Ca Mg 21 Sc Al 22 Ti Si 23 V CI Ar K 26 Fe 28 Ni Cr Mn Co Cu Zn Ga Ge As 34 Se 35 Br 36 Kr 1s¹ 1s² [He] 2s¹ [He] 2s2 [He] 2s²2p¹ [He] 2s²2p² [He] 2s²2p³ [He] 2s22p4 [He] 2s²2p5 [He] 2s²2p6 [Ne] 3s¹ [Ne] 3s2 [Ne] 3s 3p¹ [Ne] 3s23p² [Ne] 3s²3p³ [Ne] 3s23p4 [Ne] 3s²3p5 [Ne] 3s 3p6 [Ar] 4s¹ [Ar] 4s² [Ar] 3d¹4s² [Ar]3d²4s² [Ar] 3d³4s² [Ar] 3d54s¹ [Ar] 3d³4s² [Ar] 3d64s² Element 37 Rb 38 Sr 39 Y 40 Zr 41 Nb 42 Mo 43 Tc 44 Ru 45 Rh 46 Pd 47 Ag 48 Cd 49 In 50 Sn 51 Sb Te 52 53 54 55 56 57 58 59 60 61 62 63 64 I Xe Cs Ba La Ce Pr Nd Pm Sm Eu Gd [Ar]3d²4s² [Ar] 3d845² [Ar] 3d¹04s¹ [Ar] 3d¹04s2 65 Tb 66 67 [Ar] 3d¹04s²4p¹ [Ar] 3d¹04s²4p² 68 Dy Ho Er [Ar] 3d¹04s²4p³ 69 Tm [Ar] 3d¹04s²4p4 70 Yb [Ar]3d¹04s²4p5 71 Lu [Ar]3d¹04s²4p6 Configuration Z [Kr] 5s¹ [Kr] 5s² [Kr] 4d¹5s² [Kr] 4d²5s² [Kr] 4d45s¹ [kr] 4d55s¹ [kr] 4d55s² [Kr] 4d75s¹ [Kr] 4d85s1 [Kr] 4d10 [Kr] 4d105s1 [kr] 4d¹05s² [kr] 4d¹05s²5p¹ [kr] 4d¹05s25p² [Kr] 4d¹05s²5p³ [kr] 4d¹05s25p4 [Xe] 6s² [Xe] 5d¹6s² [Xe] 4f²6s² [Xe] 4f³6s² [Xe] 4f46s2 [Xe] 4f6s2 [Xe] 4f6s2 [Xe] 4f²6s² [Xe] 4f75d¹6s² [Xe] 4f%s2 [Xe] 4f106s2 [Xe] 4f¹¹6s² NRNKERKR [Xe] 4f126s2 [Xe] 4f136s2 [Xe] 4f146s2 [Xe] 4f¹45d¹6s² 72 Hf 73 Ta W 74 75 Re 76 Os 77 Element 78 79 80 81 82 83 84 85 86 87 Fr [kr] 4d¹05s²5p5 88 Ra [Kr] 4d¹05s²5p6 89 Ac [Xe] 6s¹ 90 Th 91 92 93 94 95 96 97 98 99 Ir Pt Au Hg TI Pb Bi Po At Rn Pa U Np Pu Am Cm Bk Cf Es 100 Fm 101 Md 102 No 103 Lr 104 Rf 105 Db 106 Sg Configuration [Xe] 4f¹45d²6s² [Xe] 4f145d³6s² [Xe] 4f145d46s2 [Xe] 4f145d56s2 [Xe] 4f145d6s2 [Xe] 4f¹45d²6s² [Xe] 4f¹45dº6s¹ [Xe] 4f145d106s1 [Xe] 4f145d106s2 [Xe] 4f145d6s26p* [Xe] 4f145d106s36p? [Xe] 4f145d16s?6p3 [Xe] 4f145d6s®6p* [Xe] 4f145d16s26p5 [Xe] 4f145d106s26p6 [Rn] 7s¹ [Rn] 7s² [Rn] 6d¹7s² [Rn] 6d²7s² [Rn] 5f26d¹7s² [Rn] 5f³6d¹7s² [Rn] 5f46d¹7s2 [Rn] 5f67s² [Rn] 5f77s² [Rn] 5f76d¹7s² [Rn] 5f97s2 [Rn] 5f107,2 [Rn] 5f117s2 [Rn] 5f¹27s² [Rn] 5f137,2 [Rn] 5f147s2 Rn] 5f¹46d¹7s² [Rn] 5f¹46d²7s² [Rn] 5f¹46d³7s2 [Rn] 5f¹46d¹7s²
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
a Entropy change for the expansion of a mole of Heg from V to 2V at 298 K Since helium is an ideal g...View the full answer

Answered By

Bhartendu Goyal
Professional, Experienced, and Expert tutor who will provide speedy and to-the-point solutions. I have been teaching students for 5 years now in different subjects and it's truly been one of the most rewarding experiences of my life. I have also done one-to-one tutoring with 100+ students and help them achieve great subject knowledge. I have expertise in computer subjects like C++, C, Java, and Python programming and other computer Science related fields. Many of my student's parents message me that your lessons improved their children's grades and this is the best only thing you want as a tea...
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
Calculate the entropy change, S, for the following processes. If necessary, look up required data in Appendix D. (a) The pressure of one mole of O 2 (g) is increased from P to 2P at 298 K. (b) The...
-
3. Assume that you hold a well-diversified portfolio that has an expected return of 10.0% and a beta of 1.20. You are in the process of buying 1,000 shares of Bridge Corp at $10 a share and adding it...
-
Calculate the entropy change for a process in which 3.00 moles of liquid water at 0oC is mixed with 1.00 mole of water at 100.oC in a perfectly insulated container. (Assume that the molar heat...
-
Find the maximum of f(x,y) = x + y - x - y - xy
-
School costs money. Is this an expenditure that you should have avoided? A year of tuition at a public four-year college costs about $8,655, and a year of tuition at a public two-year college costs...
-
An engineer is comparing the time to failure (in flight hours) of two different air conditioners for airplanes and wants to determine if the median time to failure for model \(\mathrm{Y}\) is longer...
-
The importance of family-friendly employee benefits
-
Richards Tree Farm Grows Up 1. Major financial management decisions involve capital budgeting, capital structure, and working capital management. Give an example of each that relates to Richards Tree...
-
Old MathJax webview Old MathJax webview need ans ASAP please KV Accounting and Business Consultants provides a variety of consulting services to a diverse range of clients. The company has three...
-
Estimate the normal boiling point of bromine, Br 2 in the following way: Determine vap H for Br 2 from data in Appendix D. Assume that vap H remains constant and that Troutons rule is obeyed. TABLE...
-
In Example 13-3, we dealt with vap H and vap S for water at 100 C. (a) Use data from Appendix D to determine values for these two quantities at 25 C. (b) From your knowledge of the structure of...
-
It is important to decide early how you intend to approach the research of your topic. To practice thinking through the initial steps of the process, select a subject of research interest from your...
-
United States HistoryBattle of Gettysburg American Civil War [1863] When and where was the Battle of Gettysburg fought?
-
United States History - United States presidential election of 1968 United States government
-
Salem witch trials American history What caused the Salem witch trials? How many people were killed during the Salem witch trials?
-
Plains Wars United States history
-
A factory has the following four major loads: A motor rated at 5 hp, 0.8 pf lagging (1 hp = 0.7457 kW). A heater rated at 1.2 kW, 1.0 pf. Ten 120-W lightbulbs. A synchronous motor rated at 1.6...
-
What are the typical record-at-a-time operations for accessing a file? Which of these depend on the current file record?
-
Sigma Corporation applies overhead cost to jobs on the basis of direct labor cost. Job V, which was started and completed during the current period, shows charges of $5,000 for direct materials,...
-
Estimated cost and operating data for three companies for the upcoming year follow: Predetermined overhead rates are computed using the following allocation bases in the three companies: Required:...
-
The following information is taken from the accounts of Latta Company. The entries in the T-accounts are summaries of the transactions that affected those accounts during the year. The overhead that...
-
XYZ Corp. applies manufacturing overhead costs to products at a budgeted indirect-cost rate of $65 per direct manufacturing labor-hour. A retail outlet has requested a bid on a special order of a...
-
What did you observe to be the major causes for the volatile week in stock trading this past week?
-
Analyze why there was underpricing or overpricing on listing price for Change Healthcare (CHNG)

Study smarter with the SolutionInn App


