Determine K c for the reaction N 2 (g) + O 2 (g) + Cl 2 (g)
Question:
Determine Kc for the reaction N2(g) + O2(g) + Cl2(g) ⇌ 2 NOCl(g), given the following data at 298 K.
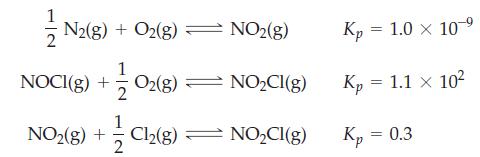
Transcribed Image Text:
N2(g) + O2(g) — NO₂(g) 102(8) NO₂Cl(g) 1 NO2(g) + Cl₂(8) ⇒ NO₂Cl(g) 2 NOCI(g) + Kp = 1.0 × 10-9 Kp = 1.1 × 102 Kp = 0.3
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (4 reviews)
To determine the equilibrium constant Kc for the given overall reaction N2g O2g Cl2g 2NOCIg Were giv...View the full answer

Answered By

Bhartendu Goyal
Professional, Experienced, and Expert tutor who will provide speedy and to-the-point solutions. I have been teaching students for 5 years now in different subjects and it's truly been one of the most rewarding experiences of my life. I have also done one-to-one tutoring with 100+ students and help them achieve great subject knowledge. I have expertise in computer subjects like C++, C, Java, and Python programming and other computer Science related fields. Many of my student's parents message me that your lessons improved their children's grades and this is the best only thing you want as a tea...
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
The equilibrium constant for the reaction N2 (g) + O,(g) ;:='02NO(g) is 1.69 x 10-3 at 2300 K. A mixture consisting of 5.0 g of nitrogen and 2.0 g of oxygen in a container of volume 1.0 dm3 is heated...
-
The equilibrium constant Kc for the reaction at 450oC is 0.159. Calculate the equilibrium composition when 1.00 mol N2 is mixed with 3.00 mol H2 in a 5.00-L vessel. N2(g) 3H2(g) 2NH3(g)
-
The molecule methylamine (CH3NH2) can act as a monodentate ligand. The following are equilibrium reactions and the thermochemical data at 298 K for reactions of methylamine and en with Cd2+ (aq); (a)...
-
On March 1, 2014, Eire Co. paid $4,800 to Big North Insurance for a one-year insurance policy. Eire Co. has a December 31 fiscal year end and adjusts accounts annually. Complete the following for...
-
What is operating leverage? What causes it? How is the degree of operating leverage (DOL) measured?
-
The accounting profession is organized into three major groups: (a) Accountants who work in non business entities, (b) Accountants who work in business entities, (c) Accountants in public practice....
-
17-12. Can personal privacy become a problem as the real and digital worlds converge with smart systems?
-
An air-standard Diesel cycle has a compression ratio of 16 and a cutoff ratio of 2. At the beginning of the compression process, air is at 95 kPa and 27C. Accounting for the variation of specific...
-
ZOOM Video Communications ZM stock currently sells for $402.20 per share. ZM just paid a dividend of $4.25. If the required rate of return on ZM stock or similar video communication companies is...
-
For the reaction 2 H 2 S(g) 2 H 2 (g) + S 2 (g), the equilibrium constant is K c = 4.20 x 10 -6 at 830 C. What are the equilibrium concentrations when 0.500 mol H 2 S is placed in an empty 1.0 L...
-
Use the following data to estimate a value of K p at 1200 K for the reaction 2 H 2 (g) + O 2 (g) 2 H 2 O(g) C(graphite) + CO2(g) CO(g) + H(g) = C(graphite) + O(g) 1 2 2 CO(g) CO(g) + HO(g) CO(g) Kc...
-
Derive the stiffness matrix of each of the systems shown in Figs. 6.19 using the indicated coordinates. 31 4 4 3k O 8(t) 2k M,(t) Rigid bar, mass= 2m C 2m x1(t) F(t) m T x2(t) F2(t) FIGURE 6.19 Rigid...
-
Refer to Figure 11.2: Is it more costly to build in Los Angeles or in Washington DC? What is the cost difference? Figure 11.2 Location Factors Costs shown in RSMeans Square Foot Costs are based on...
-
Suppose the prism in Figure P33.27 is immersed in a liquid in which the speed of light is lower than the speed of light in glass. Describe what happens to the light shown entering at normal...
-
Each year, the AICPA issues a general audit risk alert document and a number of industry audit risk alerts. If you can obtain access to a current copy of either the general alert or one of the...
-
The multieffect distillation system shown in Figure 11-4 appears to be able to cut energy use in half; however, the reduction is not this large. Explain why. Figure 11-4 F PL D, D Reflux B PH
-
Schemes 11-6E and 11-6F accomplish the same task of removing and purifying an intermediate component. a. What factors enter into the decision to use scheme \(11-6 \mathrm{~F}\) instead of \(11-6...
-
Kennewick Corp. had a beginning balance in accounts receivable of $70,000 and an ending balance of $91,000. Credit sales during the period were $598,000. Determine cash collections.
-
At 31 December 20X9, the end of the annual reporting period, the accounts of Huron Company showed the following: a. Sales revenue for 20X9, $ 2,950,000, of which one- quarter was on credit. b....
-
Thomson Company estimates that total factory overhead costs will be $600,000 for the year. Direct labor hours are estimated to be 250,000. For Thomson Company, (a) Determine the predetermined factory...
-
Lewis Company estimates that total factory overhead costs will be $200,000 for the year. Direct labor hours are estimated to be 25,000. For Lewis Company, (a) Determine the predetermined factory...
-
At the end of May, Thomson Company had completed Jobs 70 and 71. Job 70 is for 8,000 units, and Job 71 is for 10,000 units. Using the data from Practice Exercises 19-1A, 19-2A, and 19-4A, determine...
-
What is the Macaulay duration of a bond with a coupon of 6.6 percent, seven years to maturity, and a current price of $1,069.40? What is the modified duration? (Do not round intermediate...
-
"Tell me something you know today that you did not know yesterday" about 3D Animation Justify by citing 2 or more resources from this course.
-
Warrants exercisable at $20 each to obtain 50,000 shares of common stock were outstanding during a period when the average market price of the common stock was $25. Application of the treasury stock...

Study smarter with the SolutionInn App


