Use the following data to estimate a value of K p at 1200 K for the reaction
Question:
Use the following data to estimate a value of Kp at 1200 K for the reaction 2 H2(g) + O2(g) ⇌ 2 H2O(g)
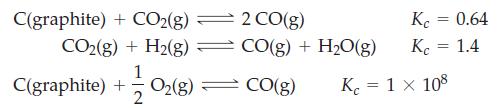
Transcribed Image Text:
C(graphite) + CO2(g) ⇒ CO₂(g) + H₂(g) = C(graphite) + O₂(g) 1 2 2 CO(g) CO(g) + H₂O(g) CO(g) Kc = 0.64 Kc = 1.4 Kc = 1 x 108
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (1 review)
To estimate the value of Kp at 1200 K for the reaction 2 H2g O2g 2 H...View the full answer

Answered By

Amit Choudhary
I'm new in this profession regarding online teaching but previously i used to teach students near my college. I am teaching on online platform since last year and got good support from the students. I'm teaching on platforms like chegg and vedantu and also at my home in free time.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
The equilibrium constant for the H2 + ½ O2 H2O reaction at 1 atm and 1200 K is KP. Use this information to determine the equilibrium constant for the following reactions: (a) at l atm H, +...
-
Use the following data to estimate ÎHof for magnesium fluoride. Mg(s) + F2(g) MgF2(s) Lattice energy First ionization energy of Mg Second ionization energy of Mg 1445 k/mol Electron affinity of...
-
The current (in amperes) at time t (in seconds) flowing in the circuit in Figure 19 is given by Kirchhoff's Law: i(t) = Cv (t) + R 1v(t) where v(t) is the voltage (in volts), C the capacitance (in...
-
Refer to the information in BE4-7 for Hébert Company. Prepare the correcting journal entries. Information in BE4-7 1. A collection of cash on account from a customer for $750 was recorded as a...
-
What is the operating breakeven point? How do changes in fixed operating costs, the sale price per unit, and the variable operating cost per unit affect it?
-
Agency Rent-A-Car Inc. rents cars to customers whose vehicles are unavailable due to accident, theft, or repair (Wheels while your car heals). The company has a fleet of more than 40,000 cars located...
-
1 In your new job as a retail store manager you decide to add mobile marketing to your promotional campaign. Describe how ads directed at connected cars could increase sales in your store.
-
Montel Companys July sales budget calls for sales of $600,000. The store expects to begin July with $50,000 of inventory and to end the month with $40,000 of inventory. Gross margin is typically 40%...
-
Crane Industrial Products Inc. is a diversified industrial-cleaner processing company. The company's Dargan plant produces two products: a table cleaner and a floor cleaner from a common set of...
-
Determine K c for the reaction N 2 (g) + O 2 (g) + Cl 2 (g) 2 NOCl(g), given the following data at 298 K. N2(g) + O2(g) NO(g) 102(8) NOCl(g) 1 NO2(g) + Cl(8) NOCl(g) 2 NOCI(g) + Kp = 1.0 10-9 Kp...
-
(A) Excess Ag(s) is added to 1.20 M Fe 3+ (aq). Given that what are the equilibrium concentrations of the species in solution? (B) A solution is prepared with [V 3+ ] = [Cr 2+ ] = 0.0100 M and [V 2+...
-
DAF Trucks is based in Oevel, in Westerlo, Belgium. It was established in 1966, and the size and facilities in the manufacturing plant have been developed gradually over the past 50 or so years with...
-
Verify the results of Eq. (14.48) for the properties of the chiral projection operators. Data from Eq. 14.48 P = P+ P+ + P = 1 P_P+ P+P = 0 Py" = y P
-
Prove that the estimating equations in (11.13) are unbiased under MCAR, but are generally biased without the stringent MCAR assumption. (x) [y - f (xt;)] = 0, i=1 (11.13)
-
Refer to Figure 11.5: Which is the most expensive subcontract for this project? How much were the costs for the general contractor's crews for item 4? Figure 11.5 Division 1 2 3 4 5 6 7 Work Gen'l...
-
a. Using observations on the change in consumption \(D C_{t}=C_{t}-C_{t-1}\) and the change in income \(D Y_{t}=\) \(Y_{t}-Y_{t-1}\) from 1959Q3 to 2015Q4, obtained from the data file cons_inc,...
-
Water at \(20^{\circ} \mathrm{C}\) flows by gravity from a large reservoir at a high elevation to a smaller one through a 35-m-long, 5-cm-diameter cast iron piping system that includes four standard...
-
During its first year of operations, Fertig Company had credit sales of $3,000,000, of which $400,000 remained uncollected at year-end. The credit manager estimates that $18,000 of these receivables...
-
Why is disclosure of depreciation or amortization methods and rates so important?
-
At the end of June, Lewis Company had completed Jobs 30 and 32. Job 30 is for 1,600 units, and Job 32 is for 1,750 units. Using the data from Practice Exercises 19-1B, 19-2B, and 19-4B, determine (a)...
-
Luek Company completed 60,000 units during the year at a cost of $900,000. The beginning finished goods inventory was 10,000 units at $140,000. Determine the cost of goods sold for 45,000 units,...
-
Suo Company completed 20,000 units during the year at a cost of $120,000. The beginning finished goods inventory was 2,500 units at $14,000. Determine the cost of goods sold for 12,000 units,...
-
En prenant un exemple de votre choix, montrer comment on value un swap de taux de change.
-
How much would you need to invest today in order to receive: a. $10,000 in 5 years at 11%? b. $11,000 in 12 years at 8%? c. $12,000 each year for 10 years at 8%? d. $12,000 at the beginning of each...
-
A company that manufactures pulse Doppler insertion flow meters uses the Straight Line method for book depreciation purposes. Newly acquired equipment has a first cost of $190,000 with a 3-year life...

Study smarter with the SolutionInn App


