Determine the values of K p from the K c values given. (a) NO4(g) K (b) 2
Question:
Determine the values of Kp from the Kc values given.
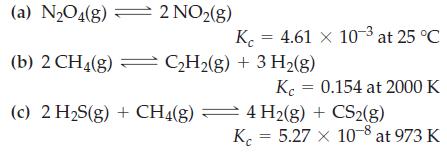
Transcribed Image Text:
(a) N₂O4(g) K (b) 2 CH4(g) = C₂H₂(g) + 3 H₂(g) (c) 2 H₂S(g) + CH4(g) 2 NO₂(g) = 4.61 x 10-3 at 25 °C Kc = 0.154 at 2000 K 4 H2(g) + CS2(g) K = 5.27 x 10-8 at 973 K
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 83% (6 reviews)
Here are the conversion factors between Kp and Kc for the given reactions Reaction Kp Kc Conversion ...View the full answer

Answered By

Arun kumar
made more than four thousand assignments
5.00+
3+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
You have been assigned the task of measuring the equilibrium constant for the reaction N 2 O 4 2NO 2 as a function of temperature. To do so, you evacuate a rigid 2-liter vessel equipped with a...
-
Consider the nanofluid of Example 2.2. Example 2.2 (a) Calculate the Prandtl numbers of the base fluid and nanofluid, using information provided in the example problem. (b) For a geometry of fixed...
-
A 0.831-g sample of SO3 is placed in a 1.00-L container and heated to 1100 K. The SO3 decomposes to SO2 and O2: At equilibrium the total pressure in the container is 1.300 atm. Find the values of Kp...
-
Because Natalie has been so successful operating Cookie Creations, Katy would like to have Natalie become her partner. Katy believes that together they will create a thriving cookie-making business....
-
Ogden Corporation has compiled the following information on a capital expenditure proposal: (1) The projected cash inflows are normally distributed with a mean of $36,000 and a standard deviation of...
-
Rubin Enterprises had the following sales-related transactions on a recent day: a. List price of services provided on credit was $18,150; terms 2/10, n/45. b. Collected $3,650 in cash for services to...
-
Describe how to establish the approximate price level using demand-oriented, cost-oriented, profit-oriented, and competition-oriented approaches.
-
Your roommate, Matt Mikan, contends that accounting contributes to most of the steps in managements decision- making process. Is your roommate correct? Explain.
-
For the current year, Electric Corporation expected to sell 42,700 industrial power cords. Fixed costs were expected to total $1,653,500; unit sales price was expected to be $4,100; and unit variable...
-
(A) Equilibrium is established in a 3.00 L flask at 1405 K for the reaction 2 H 2 S(g) 2 H 2 (g) + S 2 (g). At equilibrium, there is 0.11 mol S 2 (g), 0.22 mol H 2 (g), and 2.78 mol H 2 S(g). What...
-
(A) The reaction N 2 O 4 (g) 2 NO 2 (g) has r H = +57.2 kJ mol -1 . Will the amount of NO 2 (g) formed from N 2 O 4 (g) be greater at high or low temperatures? (B) The enthalpy of formation of NH 3...
-
Independent random samples of professional football and basketball players gave the following information (References: Sports Encyclopedia of Pro Football and Official NBA Basketball Encyclopedia)....
-
The English statistician Karl Pearson (1857-1936) introduced a formula for the skewness of a distribution. P = 3 ( x median ) s Pearson's index of skewness Most distributions have an index of...
-
You are to specify an orifice meter for measuring the flow rate of a $35^{\circ} \mathrm{API}$ distillate $(\mathrm{SG}=0.85$ ) flowing in a $2 \mathrm{in}$. sch 160 pipe at $70^{\circ} \mathrm{F}$....
-
Let $\theta$ and $\phi$ be the polar coordinates. Introduce the complex numbers $z$ and $\bar{z}$, where $$\begin{equation*} z=e^{i \phi} \tan (\theta / 2) \equiv \xi+i \eta \tag{5.393}...
-
Suppose the profit \(P\) (in dollars) of a certain item is given by \(P=1.25 x-850\), where \(x\) is the number of items sold. a. Graph this profit relationship. b. Interpret the value of \(P\) when...
-
(a) Draw a simplified ray diagram showing the three principal rays for an object located outside the focal length of a diverging lens. (b) Is the image real or virtual? (c) Is it upright or inverted?...
-
Daisey Company is a very profitable small business. It has not, however, given much consideration to internal control. For example, in an attempt to keep clerical and office expenses to a minimum,...
-
According to a New York Times columnist, The estate tax affects a surprisingly small number of people. In 2003, . . . just 1.25 percent of all deaths resulted in taxable estates, with most of them...
-
Alcoa Inc. is the worlds largest producer of aluminum products. One product that Alcoa manufactures is aluminum sheet products for the aerospace industry. The entire output of the Smelting Department...
-
Domino Foods, Inc., manufactures a sugar product by a continuous process, involving three production departmentsRefining, Sifting, and Packing. Assume that records indicate that direct materials,...
-
The chief cost accountant for Mountain Glade Beverage Co. estimated that total factory overhead cost for the Blending Department for the coming fiscal year beginning March 1 would be $546,000, and...
-
Physical Units Method, Relative Sales Value Method Farleigh Petroleum, Inc., is a small company that acquires high - grade crude oil from low - volume production wells owned by individuals and small...
-
A proposed $2.5 M investment in new equipment at a 100 MG/y M&Ms factory will save the plant $800,000/y in energy costs. Assuming an annual interest rate of 5%/y (compounded annually), and an...
-
Brief Exercise 10-7 Coronado Company obtained land by issuing 2,250 shares of its $14 par value common stock. The land was recently appraised at $103,240. The common stock is actively traded at $44...

Study smarter with the SolutionInn App


