Estimate the enthalpy of formation of HCN using bond energies from Table 10.3, data from elsewhere in
Question:
Estimate the enthalpy of formation of HCN using bond energies from Table 10.3, data from elsewhere in the text, and the reaction scheme outlined as follows.
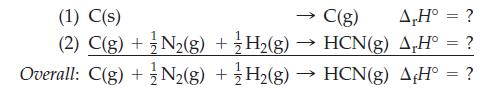
Table 10.3
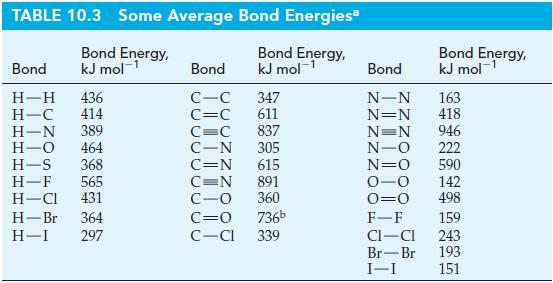
Transcribed Image Text:
C(g) A,H° = ? HCN(g) AH° = ? H₂(g) → HCN(g) AH° = ? (1) C(s) (2) C(g) + N₂(g) + H₂(g) Overall: C(g) + N₂(g) +
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
AH717 KJmol C 8 C ...View the full answer

Answered By

Carly Cimino
As a tutor, my focus is to help communicate and break down difficult concepts in a way that allows students greater accessibility and comprehension to their course material. I love helping others develop a sense of personal confidence and curiosity, and I'm looking forward to the chance to interact and work with you professionally and better your academic grades.
4.30+
12+ Reviews
21+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
Resonance energy is the difference in energy between a real moleculea resonance hybridand its most important contributing structure. To determine the resonance energy for benzene, we can determine an...
-
Using bond energies from Table, estimate the barrier to rotation around a carboncarbon double bond. To do this, consider what must happen to go from In terms of making and breaking chemical bonds;...
-
The gas-phase reaction shown, between N2 and O2, was run in an apparatus designed to maintain a constant pressure. (a) Write a balanced chemical equation for the reaction depicted and predict whether...
-
Name the types of consumer decision-making processes. List some products you have bought using each type. Have you ever bought a product on impulse? If so, describe the circumstances.
-
Lovell Computer Parts Inc. is in the process of setting a selling price on a new component it has just designed and developed. The following cost estimates for this new component have been provided...
-
Let us consider a random linear constraint where a axb,
-
The standard model of consolidation proposes that memory retrieval depends on the hippocampus during consolidation but that after consolidation is complete, retrieval involves the cortex and the...
-
Lorring Landscaping has the following data for the December 31 adjusting entries: a. Each Friday, Lorring pays employees for the current weeks work. The amount of the weekly payroll is $6,000 for a...
-
At the end of the current year, Accounts Receivable has a balance of $3,000,000; Allowance for Doubtful Accounts has a debit balance of $475,000; and Net Sales for the year total $6,500,000. Bad Debt...
-
The standard enthalpy of formation of H 2 O 2 (g) is -136 kJ mol -1 . Use this value, with other appropriate data from the text, to estimate the oxygen-to-oxygen single-bond energy. Compare your...
-
In certain polar solvents, PCl 5 undergoes an ionization reaction in which a Cl - ion leaves one PCl 5 molecule and attaches itself to another. The products of the ionization are PCl 4 + and PCl 6 -...
-
You are in a violent argument with a chartist. He claims that you are violating the fundamental laws of economics by trying to find intrinsic value. Price is determined by demand and supply, not by...
-
In Exercises 21-24, use these results from the "1-Panel-THC" test for marijuana use, which is provided by the company Drug Test Success: Among 143 subjects with positive test results, there are 24...
-
I need help for an assignment of a review on research on Virtual Education on study motivation and academic performance in university students. I am attaching a research article from a magazine to...
-
Shouldice Hospital in Canada is widely known for one thing-hernia repair! In fact, that is the only operation it performs, and it performs a great many of them. Over the past two decades this small...
-
Required information Use the following information for the Exercises below. (Algo) [The following information applies to the questions displayed below.] Ramirez Company installs a computerized...
-
Reproduced below from Farthington Supply s accounting records is the accounts receivable subledger along with selected general ledger accounts. General Ledger Accounts Receivable Dec. 3 1 / 2 2...
-
For the Y-Y circuit of Fig. 12.41, find the line currents, the line voltages, and the load voltages. 220/0 v A 10 j5 220 2-1209V 220/120 v
-
Economic feasibility is an important guideline in designing cost accounting systems. Do you agree? Explain.
-
Joint-cost allocation, process further or sell. (CMA, adapted) Sonimad Sawmill, Inc. (381), purchases logs from independent timber contractors and processes the logs into three types of lumber...
-
Joint-cost allocation. Elsie Dairy Products Corp buys one input full-cream milk, and refines it in a churning process. From each gallon of milk Elsie produces two cups (one pound) of butter and two...
-
Further processing decision (continuation of 18-30). Elsie has decided that buttermilk may sell better if it was marketed for baking and sold in pints. This would involve additional packaging at an...
-
() Y 0 0 0 0 0 0 0 1 0 1 1 0 1 0 1 1 0 1 0 1 0 1 0 1 1 1 1 1 1 1 1 0 1 1 0 0 1 1 0 0 1 1 0 0 1 1 D 0 1 1 0 1 1 1 1 0 1 0 1 7. For Exercise 2.6e implement the design in SV (there are multiple ways)...
-
Requirement. For each depreciation method prepare a depreciation schedule showing asset cost de connected dopeciation and book for the units of production method, round depreciation of unilla three...
-
Audit documentation of the evidence gathered by the auditor should meet which of the following criteria? (2 points) Workpapers are prepared in sufficient detail so that they can be given to the...

Study smarter with the SolutionInn App


