For a coordination number of four, the radius of Mn 7+ has been estimated to be 39
Question:
For a coordination number of four, the radius of Mn7+ has been estimated to be 39 pm. Estimate the charge density for the Mn7+ ion. Express your answer in C mm–3. How does this compare with the charge density of Be2+ given in Table 21.4? Would you expect the bonding in Mn2O7 to be primarily ionic or primarily covalent? Explain.
Table 21.4
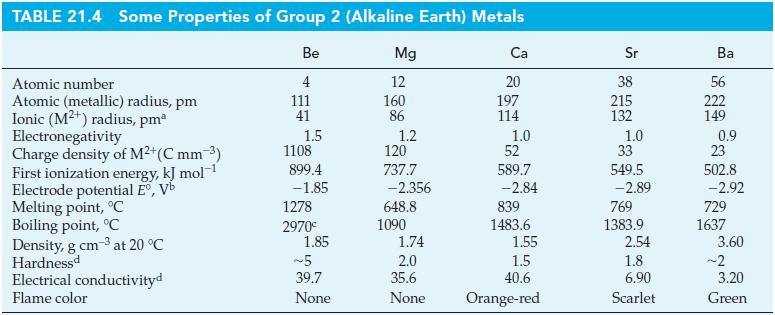
Transcribed Image Text:
TABLE 21.4 Some Properties of Group 2 (Alkaline Earth) Metals Be 4 111 41 Atomic number Atomic (metallic) radius, pm Ionic (M²¹) radius, pmª Electronegativity Charge density of M²+ (C mm ³) First ionization energy, kJ mol-¹ Electrode potential Eº, Vb Melting point, °C Boiling point, °C Density, g cm-3 at 20 °C Hardnessd Electrical conductivityd Flame color 1.5 1108 899.4 -1.85 1278 2970€ 1.85 ~5 39.7 None Mg 12 160 86 1.2 120 737.7 -2.356 648.8 1090 1.74 2.0 35.6 None Ca 20 197 114 1.0 52 589.7 -2.84 839 1483.6 1.55 1.5 40.6 Orange-red Sr 38 215 132 1.0 33 549.5 -2.89 769 1383.9 2.54 1.8 6.90 Scarlet Ba 56 222 149 0.9 23 502.8 -2.92 729 1637 3.60 ~2 3.20 Green
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 33% (3 reviews)
The charge density of an ion is calculated using the formula Charge density Charge Volume Given that ...View the full answer

Answered By

Fahmin Arakkal
Tutoring and Contributing expert question and answers to teachers and students.
Primarily oversees the Heat and Mass Transfer contents presented on websites and blogs.
Responsible for Creating, Editing, Updating all contents related Chemical Engineering in
latex language
4.40+
8+ Reviews
22+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
Find 1NF, 2NF and 3NF for the following table EMP_NUM 1003 1018 EMP_LNAME Willaker Smith BBA, MBA BBA SLS SLS EMP_EDUCATION JOB CLASS EMP DEPENDENTS DEPT CODE DEPT_NAME DEPT MANAGER EMP TITLE EMP DOB...
-
During the last few years, Jana Industries has been too constrained by the high cost of capital to make many capital investments. Recently, though, capital costs have been declining, and the company...
-
reading the following article "How Truthful are memoirs? The Pulitzer Board should answer that question soon" As you read the article, reflect on the following questions what is a "factual memoir"?...
-
The two optional steps in the accounting cycle are preparing O a worksheet and post-closing trial balances. an adjusted trial balance and a post-closing trial balance. O a post-closing trial balance...
-
Pump-It, Inc., sells weight-lifting equipment. The sales and inventory records of the company for January through March 2012 were as follows: Required: 1. Determine the amounts for ending inventory ,...
-
How does the tax benefit rule apply in the following cases? a. In 2014, the Orange Furniture Store, an accrual method taxpayer, sold furniture on credit for $1,000 to Sammy. The cost of the furniture...
-
BRCA Gene In the general population, one woman in eight will develop breast cancer. Research has shown that approximately 1 woman in 600 carries a mutation of the BRCA gene. About 6 out of 10 women...
-
On July 1, 2013, Ross-Livermore Industries issued nine-month notes in the amount of $400 million. Interest is payable at maturity. Required: Determine the amount of interest expense that should be...
-
A company reports the following information regarding its inventory. Beginning inventory: cost is $80,000; retail is $130,000 Net purchases: cost is $65,000; retail is $120,000 Sales at retail:...
-
Nearly all mercury(II) compounds exhibit covalent bonding. Mercury(II) chloride is a covalent molecule that dissolves in warm water. The stability of this compound is exploited in the determination...
-
Several transition metal ions are found in cation group 3 of the qualitative analysis scheme outlined in Figure 18-7. At one point in the separation and testing of this group, a solution containing...
-
From the data in Table 14-1 in the text calculate the average annual inflation rate of first class postage as measured by the LCI for the following years: In Table 14.1 (a) End of 1970 to end of 1979...
-
Consider a piston with an orifice in a cylinder filled with a fluid of viscosity \(\mu\) as shown in Fig. 1.106. As the piston moves in the cylinder, the fluid flows through the orifice, giving rise...
-
Add a function to SmallWorld that computes the global clustering coefficient of a graph. The global clustering coefficient is the conditional probability that two random vertices that are neighbors...
-
Show that the generators of the algebra (33.8) are related by parity. For a Dirac wavefunction the action of parity is $P \psi(\boldsymbol{x}, t) P^{-1}=\gamma_{0} \psi(-\boldsymbol{x}, t)$, up to a...
-
Extend the algorithm you designed for Exercise 6.2 so that it can evaluate positions that are nonterminalin other words, positions where the game has not yet finished. Your score should be positive...
-
In addition to tanh, another s-shaped smooth function, the logistic sigmoid function y=1 / (1+exp(x)), is commonly used as an activation function in neural networks. A common way to implement them in...
-
Let f: G H be a group homomorphism onto H. If G is a cyclic group, prove that H is also cyclic.
-
A certain Christmas tree ornament is a silver sphere having a diameter of 8.50 cm. Determine an object location for which the size of the reflected image is three-fourths the size of the object. Use...
-
(a) What is a ledger? (b) Why is a chart of accounts important?
-
What is a trial balance and what are its purposes?
-
Kevin Haden is confused about how accounting information flows through the accounting system. He believes information flows in this order: (a) Debits and credits are posted to the ledger. (b)...
-
Physical Units Method, Relative Sales Value Method Farleigh Petroleum, Inc., is a small company that acquires high - grade crude oil from low - volume production wells owned by individuals and small...
-
A proposed $2.5 M investment in new equipment at a 100 MG/y M&Ms factory will save the plant $800,000/y in energy costs. Assuming an annual interest rate of 5%/y (compounded annually), and an...
-
Brief Exercise 10-7 Coronado Company obtained land by issuing 2,250 shares of its $14 par value common stock. The land was recently appraised at $103,240. The common stock is actively traded at $44...

Study smarter with the SolutionInn App


