For the alkaline Leclanch cell. (a) write the overall cell reaction. (b) Determine E cell for that
Question:
For the alkaline Leclanché cell.
(a) write the overall cell reaction.
(b) Determine E°cell for that cell reaction.
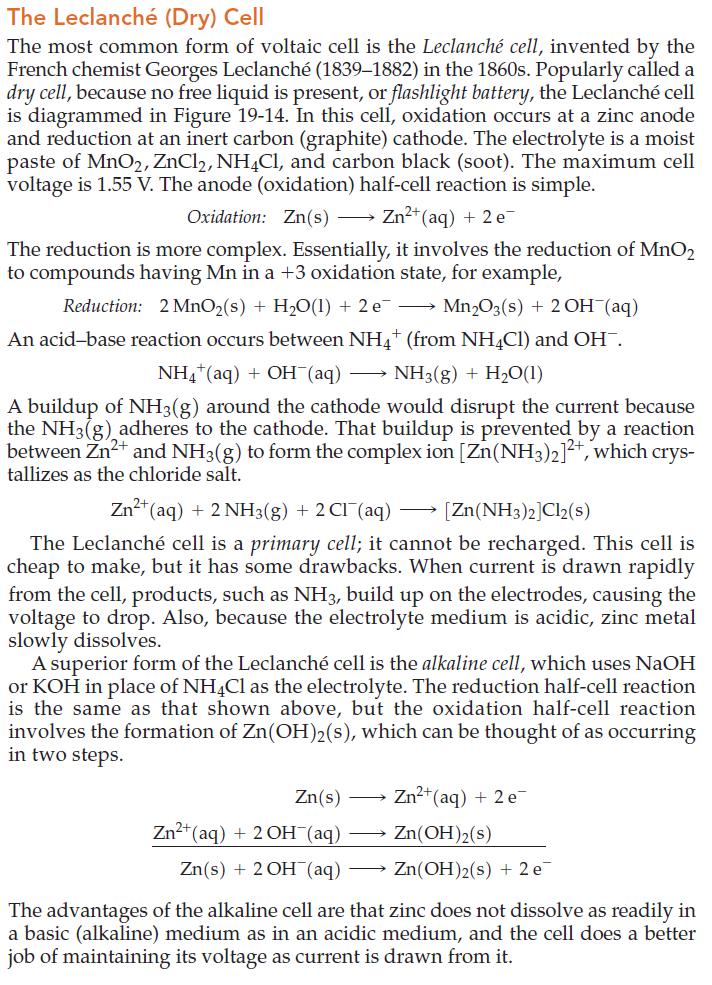
Figure 19.14
Transcribed Image Text:
The Leclanché (Dry) Cell The most common form of voltaic cell is the Leclanché cell, invented by the French chemist Georges Leclanché (1839-1882) in the 1860s. Popularly called a dry cell, because no free liquid is present, or flashlight battery, the Leclanché cell is diagrammed in Figure 19-14. In this cell, oxidation occurs at a zinc anode and reduction at an inert carbon (graphite) cathode. The electrolyte is a moist paste of MnO2, ZnCl2, NH4Cl, and carbon black (soot). The maximum cell voltage is 1.55 V. The anode (oxidation) half-cell reaction is simple. Oxidation: Zn(s) →→→ Zn²+ (aq) + 2 e The reduction is more complex. Essentially, it involves the reduction of MnO2 to compounds having Mn in a +3 oxidation state, for example, Reduction: 2 MnO₂ (s) + H₂O(1) + 2 e¯ Mn₂O3(s) + 2OH(aq) An acid-base reaction occurs between NH4+ (from NH4Cl) and OH¯. NH4+ (aq) + OH(aq) NH3(g) + H₂O(1) A buildup of NH3(g) around the cathode would disrupt the current because the NH3(g) adheres to the cathode. That buildup is prevented by a reaction between Zn²+ and NH3(g) to form the complex ion [Zn(NH3)2]²+, which crys- tallizes as the chloride salt. → Zn²+ (aq) + 2NH3(g) + 2Cl(aq) [Zn(NH3)2]Cl2(s) The Leclanché cell is a primary cell; it cannot be recharged. This cell is cheap to make, but it has some drawbacks. When current is drawn rapidly from the cell, products, such as NH3, build up on the electrodes, causing the voltage to drop. Also, because the electrolyte medium is acidic, zinc metal slowly dissolves. A superior form of the Leclanché cell is the alkaline cell, which uses NaOH or KOH in place of NH4Cl as the electrolyte. The reduction half-cell reaction is the same as that shown above, but the oxidation half-cell reaction involves the formation of Zn(OH)2(s), which can be thought of as occurring in two steps. Zn(s) Zn²+ (aq) + 2 e Zn²+ (aq) + 2OH(aq) Zn(OH)2(s) Zn(s) + 2OH(aq) → Zn(OH)2(s) + 2 e The advantages of the alkaline cell are that zinc does not dissolve as readily in a basic (alkaline) medium as in an acidic medium, and the cell does a better job of maintaining its voltage as current is drawn from it.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (4 reviews)

Answered By

Anjali Arora
Having the experience of 16 years in providing the best solutions with a proven track record of technical contribution and appreciated for leadership in enhancing team productivity, deliverable quality, and customer satisfaction. Expertise in providing the solution in Computer Science, Management, Accounting, English, Statistics, and Maths.
Also, do website designing and Programming.
Having 7 yrs of Project Management experience.
100% satisfactory answers.
5.00+
3+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
Refer to the discussion of the Leclanch cell. (a) Combine the several equations written for the operation of the Leclanch cell into a single overall equation. (b) Given that the voltage of the...
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
Use the table below to find: (fog)(-10) = (fof) (11) = X -10 f(x) -1 g(x) 4 -1 -7 -4 -7 11 (gof)(-4)= (gog)(7) = 4 7 15 4 7 -4 11 -4 15 7 -1 15 -10 -7 -10 11
-
The general term that refers to the tendency of a parcel of air to either remain in place or change its initial position is ________. a. adiabatic b. conditional instability c. stasis d. stability
-
How does the sales force relate to company organization? To channels of distribution?
-
When did unions grow the fastest? Why?
-
2. Locate the Web site for the municipality in which your college or university is located. List one major event that has occurred recently and will be recorded in the accounting records. What fund...
-
Jays Bikes is a family-owned and -operated business that stocks a wide range of bikes designed to fit the needs of professional riders, your childs first bike, and everything in between. The business...
-
Question 44 3 pts Determine the loan amount given the following: Project value: $11,000,000 Term: 30 years Interest Rate:15.9% Investment Capital Requirement $900,000 Loan to Value Ratio: 88% Cap...
-
Show that the oxidation of Cl - (aq) to Cl 2 (g) by Cr 2 O 7 2- (aq) in acidic solution, with reactants and products in their standard states, does not occur spontaneously. Explain why it is still...
-
Derive a balanced equation for the reaction occurring in the cell: (a) If E cell = 1.21 V, calculate r G and the equilibrium constant for the reaction. (b) Use the Nernst equation to determine the...
-
Average expenses for full-time resident college students at 4-year institutions during the 2015 2016 academic year are shown in the table. List the elements of each set. The set of expenses that are...
-
How do socio-cognitive mechanisms, such as social identity theory and self-categorization theory, contribute to the formation and maintenance of organizational culture ?
-
How do you Sales Forecast and an Expense forecast for future years?
-
2. Do you really think the Bono case described in Ch. 2 is a genuine ethical conflict? Explain. 6. Describe the ethical issue in the Siemens case
-
How do I calculate using the SPC method if my key metric is time
-
Labor Standards: Where Do They Belong on the International Trade Agenda? Author(s): Drusilla K. Brown Link. https://viu.summon.serialssolutions.com/?#!/search?....
-
Amounts of the assets and liabilities of Maxwell Banking Company, as of December 31, 2008, are given as follows. Also included are revenue and expense figures for the year ended on that date (amounts...
-
Linda Lopez opened a beauty studio, Lindas Salon, on January 2, 2011. The salon also sells beauty supplies. In January 2012, Lopez realized she had never filed any tax reports for her business and...
-
Power Serve Company expects to operate at 85% of productive capacity during April. The total manufacturing costs for April for the production of 30,000 batteries are budgeted as follows: Direct...
-
Roadworthy Tire and Rubber Company has capacity to produce 170,000 tires. Roadworthy presently produces and sells 130,000 tires for the North American market at a price of $90 per tire. Roadworthy is...
-
MyPhone Inc. uses the total cost concept of applying the cost-plus approach to product pricing. The costs of producing and selling 5,000 units of cellular phones are as follows: MyPhone desires a...
-
crane Inc. common chairs currently sell for $30 each. The firms management believes that it's share should really sell for $54 each. If the firm just paid an annual dividend of two dollars per share...
-
Determine the simple interest earned on $10,000 after 10 years if the APR is 15%
-
give me an example of 10 transactions from daily routine that we buy and put for me Liabilities + Owners' Equity + Revenues - Expenses

Study smarter with the SolutionInn App


