Four atoms and/or ions are sketched below in accordance with their relative atomic and/or ionic radii. Which
Question:
Four atoms and/or ions are sketched below in accordance with their relative atomic and/or ionic radii.
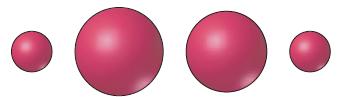
Which of the following sets of species are compatible with the sketch? Explain.
(a) C, Ca2+, Cl-, Br-;
(b) Sr, Cl, Br-, Na+;
(c) Y, K, Ca, Na+;
(d) Al, Ra2+, Zr2+, Mg2+;
(e) Fe, Rb, Co, Cs.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (3 reviews)
The sketch shows four atomsions with increasing radii from left to right Th...View the full answer

Answered By

Somshukla Chakraborty
I have a teaching experience of more than 4 years by now in diverse subjects like History,Geography,Political Science,Sociology,Business Enterprise,Economics,Environmental Management etc.I teach students from classes 9-12 and undergraduate students.I boards I handle are IB,IGCSE, state boards,ICSE, CBSE.I am passionate about teaching.Full satisfaction of the students is my main goal.
I have completed my graduation and master's in history from Jadavpur University Kolkata,India in 2012 and I have completed my B.Ed from the same University in 2013. I have taught in a reputed school of Kolkata (subjects-History,Geography,Civics,Political Science) from 2014-2016.I worked as a guest lecturer of history in a college of Kolkata for 2 years teaching students of 1st ,2nd and 3rd year. I taught Ancient and Modern Indian history there.I have taught in another school in Mohali,Punjab teaching students from classes 9-12.Presently I am working as an online tutor with concept tutors,Bangalore,India(Carve Niche Pvt.Ltd.) for the last 1year and also have been appointed as an online history tutor by Course Hero(California,U.S) and Vidyalai.com(Chennai,India).
4.00+
2+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
Use the electronegativity values in Figure 1.14 to predict which of the indicated bonds in each of the following sets is more polar. Tell the direction of the polarity in each. (a) ClOCH 3 or ClOCl...
-
Use the electro negatively table (Figure) to predict which bond in each of the following sets is more polar, and indicate the direction of bond polarity for each compound. (a) H3C ? C1 OR C1 ? C1 (b)...
-
(a) Which of the following charts below shows the general periodic trends for each of the following properties of the main-group elements (you can neglect small deviations going either across a row...
-
The following summarized statement of profit or loss has been extracted from the financial statements of Gwembe Mining Corporation, a Zambian resident company which engaged in open cast mining...
-
Management of Rivers Co. anticipates that its year-end balance sheet will show current assets of $12,639 and current liabilities of $7,480. Management is considering paying $3,850 of accounts payable...
-
Should financial markets be subject to more or less regulation if we are to avoid another financial crisis of the scale of that witnessed between 2007 and 2009? Justify your answer.
-
p. 510 What are some of the more common organizational forms that an organization might adopt for its structure?
-
The Ventron Engineering Company has just been awarded a $2 million development contract by the U.S. Army Aviation Systems Command to develop a blade spar for its Heavy Lift Helicopter program. The...
-
1. Calculate the MKC financial ratios using EXCEL and the template provided in A. Take all calculations to two decimal places. "A" 1 of 2 2019 2018 2017 account 555.1 trade accounts receivable, net...
-
Which of the following ions are likely to be found in chemical compounds: Na 2+ , Li + , Al 4+ , F 2- , or Te 2- ? Explain briefly.
-
Sketch a periodic table that would include all the elements in the main body of the table. How many numbers wide would the table be?
-
Give the major product(s) of each of the following reactions. (a) (b) (c) (d) (e) (f) OH Conc. HBr
-
Solve the Differential equation. xydx+dy=0
-
What is Hue and saturation?
-
Explain traversing on the following parcel.Provide one numerical example.
-
Explore the role of post-occupancy evaluation in commercial and industrial architecture. How do architects use feedback from building users to improve future designs?
-
Analyze the impact of sustainable construction on biodiversity and ecosystem services. How can construction practices be adapted to minimize impacts on local ecosystems and enhance biodiversity?
-
Consider a network in which all nodes are connected to three other nodes. In a single time step, a node can receive all transmitted broadcast packets from its neighbors, duplicate the packets, and...
-
In Problem use absolute value on a graphing calculator to find the area between the curve and the x axis over the given interval. Find answers to two decimal places. y = x 3 ln x; 0.1 x 3.1
-
Mason Paper Company (MPC) manufactures commodity grade papers for use in computer printers and photocopiers. MPC has reported net operating losses for the last two years due to intense price pressure...
-
Meiji Isetan Corp. of Japan has two regional divisions with headquarters in Osaka and Yokohama. Selected data on the two divisions follow (in millions of yen, denoted by): Required: 1. For each...
-
Selected operating data for two divisions of Outback Brewing, Ltd., of Australia are given below (the currency is the Australian dollar, denoted here as $): Required: 1. Compute the rate of return...
-
1. It costs $250 more per month to lease a hybrid (which gives 70mpg) than a non-hybrid (which gives 25mpg). The lease is for 3 years. How much must gas cost per gallon if you choose the hybrid...
-
The stock of Lead Zeppelin, a metal manufacturer, currently sells for $80 and has an annual standard deviation of 35 percent. The risk-free rate is 4 percent. What is the value of a put option with a...
-
PLEASE ANSWER (a) through (i) D E E F B 4.5 4 3.5 6 4 00 0 5 4 O O 6 O 00 6 9 240 360 270 2160 180 1440 210 1680 1920 2880 150 0 90 0 0 0 0 120 600 0 150 90 90 120 120 0 750 450 450 480 600 930 1800...

Study smarter with the SolutionInn App


