Use the electro negatively table (Figure) to predict which bond in each of the following sets is
Question:
Use the electro negatively table (Figure) to predict which bond in each of the following sets is more polar, and indicate the direction of bond polarity for each compound.
(a) H3C ? C1 OR C1 ? C1
(b) H3C ? H OR H ? C1
(c) HO ? CH3 OR (CH3)3Si ? CH3
(d) H3C ? Li OR Li ? OH
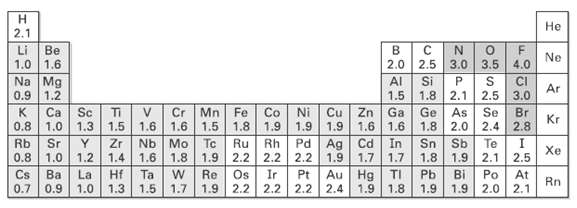
Transcribed Image Text:
Не 2.1 Li Be 1.0 1.6 B. 2.0 Ne 2.5 3.0 2.5 3.0 3.5 | 4.0 Na Mg 0.9 1.2 Ca 0.8 CI Ar Al 1.5 1.8 2.1 Si Ge As Ni Cr Mn Zn Ga Se Cu Br Kr Sc Ti 1.5 1.3 1.6 1.6 Zr Nb Mo 1.2 1.4 La Hf 1.5 1.7 1.0 1.3 Fe Co 1.8 1.9 Ru Rh Tc 1.6 1.6 In 1.7 Hg| TI 1.9 1.8 1.8 2.0 2.4 2.8 1.0 Rb 1.5 1.9 1.9 Pd Ag 1.6 1.8 | 1.9 2.2 | 2.2 2.2 Sr 0.8 Cd Sb Sn 1.8 Xe Te 1.0 Cs 1.7 2.5 1.9 2.1 1.9 Ba 0.7 0.9 Bi Po 1.9 2.0 2.1 1.9 Pb At Rn Re 1.9 Pt Au Ta Os Ir 2.2 | 2.2 2.2 2.4
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 76% (21 reviews)
a H3C CI 0 b More p...View the full answer

Answered By

Vinay Singh
I am master of science in physics and master of educationand have teaching experience of 18years.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Without using Fig. 13.3, predict which bond in each of the following groups is the most polar. a. COF, SiOF, GeOF b. POCl, SOCl c. SOF, SOCl, SOBr d. TiOCl, SiOCl, GeOCl e. COH, SiOH, SnOH f. AlOBr,...
-
Predict which substance in each of the following pairs would have the greater intermolecular forces. a. CO2 or OCS b. SeO2 or SO2 c. CH3CH2CH2NH2 or H2NCH2CH2NH2 d. CH3CH3 or H2CO e. CH3OH or H2CO
-
Which compound in each of the following pairs would have the higher boiling point? Explain your answers. (a) (b) (c) (d) (e) (f) (g) (h) Hexane, CH3(CH2)4CH3, or nonane, CH3(CH2)7CH3 (i) OH or HO OH...
-
A teacher has just given an algebra exam. What are some of the statistics she could compute?
-
On December 28, 2016, Kramer sells 150 shares of Lavender, Inc. stock for $77,000. On January 10, 2017, he purchases 100 shares of the same stock for $82,000. a. Assuming that Kramer's adjusted basis...
-
Suppose that the following fraction defective has been found in successive samples of size 100 (read down): (a) Using all the data, compute trial control limits for a fraction- defective control...
-
Using ANES data, carry out an ANOVA using the Muslims Feeling Thermometer. Compare the groups on the original religion variable regarding the Bible (whether or not it is the word of God). Explain...
-
Doxey Company purchased a machine on January 1, 2011, for $1,250,000 for the express purpose of leasing it. The machine was expected to have a 9-year life from January 1, 2011, no salvage value , and...
-
Garnet Service anticipates the following sales revenue over a five - month period: View the sales data.LOADING... View additional information.LOADING... How much cash will be collected in January? In...
-
In this exercise, you modify one of the Projected Sales applications from this chapters Apply lesson. Use Windows to make a copy of the Projected Sales Solution-ForNext folder. Rename the copy...
-
Identify the most electronegative element in each of the following molecules: (a) CH2FC1 (b) FCH2CH2CH2Br (c) HOCH2CH2NH2 (d) CH3OCH2Li
-
Which of the following molecules has a dipole moment? Indicate the expected direction ofeach. (b) (d) (c) (a)
-
TEXPAK (Pakistan) in the United Kingdom. TEXPAK is a Pakistani-based textile firm that is facing increasing competition from other manufacturers in emerging markets selling in Europe. All garments...
-
A program X running on processor A has a global CPI of 2 and a clock frequency of 2 GHz. The same program X running on processor B has a global CPI of 5 and a clock frequency of 5 GHz. what processor...
-
20 cm Room (3) 20 cm + 1 D=10 cm + 20 cm [ 10 10 cm Figure 7 (d) Using configuration factor formulae given in Figures 7(a), 7(b) and 7(c) Calculate configuration factor F12 in Figure 7(d) treating...
-
2. Let P(3,2,1),Q(2,1,c) and R(c,1,0) be points in R3. (a) Use the cross product definition to find the area of triangle PQR in terms of c. (b) For what values of c (if any) is PQR a right triangle?
-
Find and classify the discontinuities of the following function as removable or nonremovable. If a classification has no discontinuities, write None for your answer. Answer 03023 Hawks Learning A(x)=...
-
Do you see a parallel between the evolution of goals in economics and the move from Corporate Social Responsibility (CSR) to environmental, social, and governance (ESG), ? If so, please explain...
-
Insert the missing word/s: Any audit objective must be linked directly into the organizations own objectives (or mission). The starting place for setting audits role is to isolate what the . . . . ....
-
Imagine that your best friend knows you are taking a psychology course and wonders what psychology is all about. How would you define psychology for your friend? Write an essay on the discipline of...
-
Use the Estimation tool in Aspen Plus to estimate the physical properties of methyl vinyl ketone (MKK) after entering structure using the Molecular Structure tool.
-
Draw a Newman projection of the highest-energy conformation of 2,3-dimethylbutane about the C2-C3 bond.
-
Draw the two chair conformations for ethyl cyclohexane which is more stable.
-
Which of these compounds will have more of the conformation with the substituent on the cyclohexane ring axial present at equilibrium? CH,CH3 CI CH3 Ph CH,CH, b) or or or
-
Compute the value of ordinary bonds under the following circumstances assuming that the coupon rate is 0.06:(either the correct formula(s) or the correct key strokes must be shown here to receive...
-
A tax-exempt municipal bond has a yield to maturity of 3.92%. An investor, who has a marginal tax rate of 40.00%, would prefer and an otherwise identical taxable corporate bond if it had a yield to...
-
Please note, kindly no handwriting. Q. Suppose a 3 year bond with a 6% coupon rate that was purchased for $760 and had a promised yield of 8%. Suppose that interest rates increased and the price of...

Study smarter with the SolutionInn App


