Which of the following molecules has a dipole moment? Indicate the expected direction ofeach. (b) (d) (c)
Question:
Which of the following molecules has a dipole moment? Indicate the expected direction ofeach.
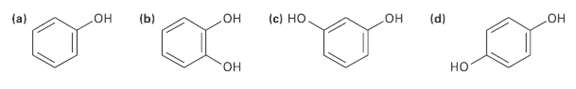
Transcribed Image Text:
(b) (d) (c) Но Он Он Он (a) но Он но
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 76% (13 reviews)
a b CI ...View the full answer

Answered By

User L
I am a proficient writer with vast experience in medicine, nursing, biochemistry, microbiology, biology chemistry, accounting, economics, mathematics, statistics, actuarial science, psychology, sociology, philosophy, and social science. I deliver papers which are original, free from plagiarism, free from grammatical errors and high-quality in time
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Which of the following molecules has the largest a value: CH4, F2, C6H6, Ne?
-
Which of the following molecules would you expect to be aromatic? (a) (b) (c) (d) (e) (f) (g) (h) (i) (j) (k) (l) N+
-
The PF3 molecule has a dipole moment of 1.03 D, but BF3 has a dipole moment of zero. How can you explain the difference?
-
When a cosmetic manufacturer tests the market to determine how many women will buy eyeliner that has been tested for safety without subjecting animals to injury, is it involved in a descriptive...
-
Tyneka inherited 1,000 shares of Aqua, Inc. stock from Joe. Joe's basis was $35,000, and the fair market value on July 1, 2016 (the date of death) was $45,000. The shares were distributed to Tyneka...
-
Consider an X control chart with UCL = 24.802, LCL = 23.792, and n = 3. Suppose that the mean shifts to 24.2. (a) What is the probability that this shift is detected on the next sample? (b) What is...
-
Using ANES data, carry out an ANOVA that will allow you to see if you can claim that the four age categories differ (use the recoded age variable that has four age categories) on the Election...
-
Kaelea, Inc., has no debt outstanding and a total market value of $70,000. Earnings before interest and taxes, EBIT, are projected to be $6,000 if economic conditions are normal. If there is strong...
-
! Required information Problem 10-56 (LO 10-2, LO 10-3) (Algo) [The following information applies to the questions displayed below.] AMP Corporation (calendar-year-end) has 2020 taxable income of...
-
The density of a uniform cylinder s determined by measuring its mass m, length I and diameter d. Calculate the density (in kg m-3) and its error from the following values m = 47.36 + 0.01 g. 1- 15.28...
-
Use the electro negatively table (Figure) to predict which bond in each of the following sets is more polar, and indicate the direction of bond polarity for each compound. (a) H3C ? C1 OR C1 ? C1 (b)...
-
(a) The H C1 bond length is 136 pm. What would the dipole moment of HC1 be if the molecule were 100% ionic, H+ C1-? (b) The actual; dipole moment HC1 is 1.08 D. What is the percent ionic character...
-
In Fig. 2-2, three process states are shown. In theory, with three states, there could be six transitions, two out of each state. However, only four transitions are shown. Are there any circumstances...
-
Manufacturing company produces $3800 worth of products weekly. If the cost of raw materials to make this product is $400, and the labour cost is $360, calculate the productivity.
-
1-You are a very well-recognized professional in your area, with many years of experience solving international conflicts. There is a company in the middle of two European countries that are fighting...
-
Find the solution u = u(x,y) of the following problem on the set R. u du - 4, (1.4) Ju(0,y) =3y, u(x, 0) = 0. (1.5) ay
-
Scenario A Sports Club 10 Highfield Sports Club has organised a fundraising event. 300 tickets have been sold at a price of $2.50 each. Money taken at the event Percentage of money (E) taken (96)...
-
Shamrock Investments has three divisions (Green, Clover, Seamrog) organized for performance evaluation purposes as investment centers. Each division's required rate of return for purposes of...
-
Which statement is least appropriate? A risk survey necessitates discussion with middle management and involves: a. A definition of the audit unit. b. An assessment of the relative risks inherent in...
-
How does health insurance risk differ from other types of insurance risk (e.g., automobile or homeowners insurance)? What is the difference between cost sharing and cost shifting? Is retiree health...
-
A gas is continuously passed through an adiabatic turbine at the rate of 2 mol/s. Its initial temperature is 600 K, its initial pressure is 5 bar and its exiting pressure is 1 bar. Determine the...
-
Bromine is larger than chlorine, yet the two atoms have identical axial destabilization energies. Explain.
-
Draw the stereo isomers of these compounds: (a) 1, 3-Dimethyleyclohexane (b) 1, 2-Diethylcycloproane (c) 1-Chloro-3-methylcyclopentane
-
Draw both chair conformations of trans-1, 3-dimethyl cyclohexane indicate whether each methyl group is axial or equatorial.
-
Be prepared to explain the texts comprehensive To illustrate the issues related to interest capitalization, assume that on November 1, 2016, Shalla Company contracted Pfeifer Construction Co. to...
-
On April 1, 2020. Indigo Company received a condemnation award of $473,000 cash as compensation for the forced sale of the company's land and building, which stood in the path of a new state highway....
-
The market price of a stock is $24.55 and it is expected to pay a dividend of $1.44 next year. The required rate of return is 11.23%. What is the expected growth rate of the dividend? Submit Answer...

Study smarter with the SolutionInn App


