Use the electronegativity values in Figure 1.14 to predict which of the indicated bonds in each of
Question:
Use the electronegativity values in Figure 1.14 to predict which of the indicated bonds in each of the following sets is more polar. Tell the direction of the polarity in each.
(a) ClOCH3 or ClOCl
(b) HOCH3 or HOCl
(c) HOOCH3 or (CH3)3SiOCH3
Fig 1.14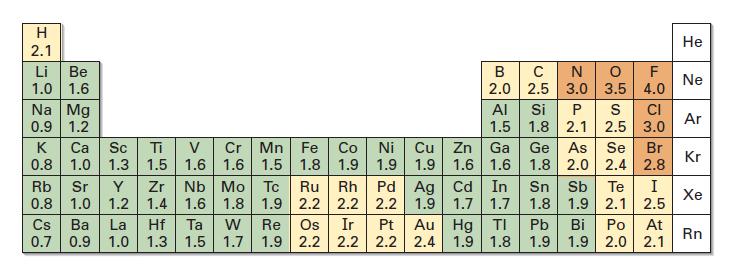
Transcribed Image Text:
H 2.1 Li Be 1.0 1.6 Na Mg 0.9 1.2 Cr Mn Fe Co Ni Cu 1.6 1.6 1.5 1.8 1.9 1.9 1.9 K Ca Sc Ti V 0.8 1.0 1.3 1.5 Rb Sr Y Zr 0.8 1.0 1.2 1.4 Cs Ba La Hf 0.7 0.9 1.0 1.3 B C N O F 2.0 2.5 3.0 3.5 4.0 Ta W 1.5 1.7 AI 1.5 Zn Ga 1.6 1.6 1.9 1.7 1.7 Nb Mo Tc Ru Rh Pd Ag Cd In 1.6 1.8 1.9 2.2 2.2 2.2 Re Os Ir Pt 1.9 2.2 2.2 2.2 Au Hg ΤΙ 2.4 1.9 1.8 Si P S CI 1.8 2.1 2.5 3.0 Ge As 1.8 2.0 2.4 Se Br 2.8 Sn Sb Te 1.8 1.9 2.1 Pb 1.9 I 2.5 Bi Po At 1.9 2.0 2.1 He Ne Ar Kr Xe Rn
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 71% (7 reviews)
To predict the polarity of each bond we need to compare the electronegativity values of the atoms involved in the bond The more significant the differ...View the full answer

Answered By

Surendar Kumaradevan
I have worked with both teachers and students to offer specialized help with everything from grammar and vocabulary to challenging problem-solving in a range of academic disciplines. For each student's specific needs, I can offer explanations, examples, and practice tasks that will help them better understand complex ideas and develop their skills.
I employ a range of techniques and resources in my engaged, interesting tutoring sessions to keep students motivated and on task. I have the tools necessary to offer students the support and direction they require in order to achieve, whether they need assistance with their homework, test preparation, or simply want to hone their skills in a particular subject area.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Use the electro negatively table (Figure) to predict which bond in each of the following sets is more polar, and indicate the direction of bond polarity for each compound. (a) H3C ? C1 OR C1 ? C1 (b)...
-
In Example 11 on ESP, John Doe had to predict which of five numbers was chosen in each of three trials. Doe did not actually have ESP. Explain why this experiment satisfies the three conditions for...
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
Show that for an integer n > 2, the period of the decimal expression for the rational number is at most n - 1. Find the first few values of n for which the period of - is equal ton- 1. Do you notice...
-
The following information pertains to United Ways, a private voluntary health and welfare organization, for the year ended December 31, 20X3. Balances in net assets at January 1, 20X3: Unrestricted...
-
Air enters an adiabatic nozzle steadily at 400kPa, 200oC and 35m/s and leaves at 150kPa and 180 m/s. The inlet area of the nozzle is 75cm2. Determine (a) The mass flow rate. (b) The exit temperature...
-
Presentation of comprehensive income must be reported under IFRS in: (a) the statement of stockholders equity. (b) the income statement ending with net income. LO15 (c) the notes to the financial...
-
Two point charges, Q1 = - 25μC and Q2 = + 50μC, are separated by a distance of 12cm. The electric field at the point P (See Fig. 16-55) is zero. How far from Q1 is P? 12 cm -25 Q2 P. +50
-
A company has a 1 3 % WACC and is considering two mutually exclusive investments ( that cannot be repeated ) with the following cash flows: 0 1 2 3 4 5 6 7 Project A - $ 3 0 0 - $ 3 8 7 - $ 1 9 3 - $...
-
1-Based on the data from the case use the frameworks from the chapter and analyse the resources and capabilities of Rocket Internet: a. What are its resources and capabilities? b. What are its...
-
The following model is that of aspartame, C 14 H 18 N 2 O 5 , known commercially under many names, including NutraSweet. Only the connections between atoms are shown; multiple bonds are not...
-
Fill in any unshared electrons that are missing from the following linebond structures: (a) H3C CH3 Dimethyl disulfide (b) H3C NH Acetamide (c) H3C Acetate ion
-
(a) Tia Kim believes that the analysis of financial statements is directed at two characteristics of a company: liquidity and profitability. Is Tia correct? Explain. (b) Are short-term creditors,...
-
You are an external auditor in a firm that undertakes the audit of Canadian Life and Mutual (CLM), a large, Montreal-based financial institution. CLM relies heavily on its computer-based information...
-
You need to temporarily increase the feed rate to an existing column without flooding. Since the column is now operating at about \(90 \%\) of flooding, you must vary some operating parameter. The...
-
Consider, again, the clothing data set. Obtain the three summary plots of the sample cross-correlations for lags 1 to 21.
-
Based on the dangling-else discussion in Exercise 3.27, modify the following code to produce the output shown. Use proper indentation techniques. You must not make any additional changes other than...
-
Consider the random process \(U(t)=A\), where \(A\) is a random variable uniformly distributed on \((-1,1)\). (a) Sketch some sample functions of this process. (b) Find the time autocorrelation...
-
During the 1970s, many women changed their minds about whether they would leave the labor force after marrying and having children or whether they would be in the labor force most of their adult...
-
B made an issue of 150,000 $1 ordinary shares at a premium of 20% the proceeds of which is received by cheque. What is the correct journal to record this? A. Bank Share capital Share premium B. Bank...
-
a. Calculate the ratios of the different kinds of protons in a compound with an integral ratio of 6 : 4 : 18.4 (going from left to right across the spectrum). b. Determine the structure of a compound...
-
The two hydrogens of a methylene group adjacent to an asymmetric carbon are not equivalent hydrogens because they are in different environments due to the asymmetric carbon. (You can verify this...
-
In a reaction called the Birch reduction, benzene can be partially reduced to 1,4-cyclohexadiene by an alkali metal (Na, Li, or K) in liquid ammonia and a low-molecular-weight alcohol. Propose a...
-
Selected comparative financial statement data for DAS inc. Balance Sheet (En milliers de dollars) 2017 2018 Assets Assets CT - Cash 41.63 47.5 - Accounts Receivable 64.2 72.6 - inventories 969.7...
-
please help!! One chance at turning in!!! 16 rows! I'd highly appreicate it I am unsure what information you need... I provided all Current Attempt in Progress Mike Greenberg opened Grouper Window...
-
Blue Ridge Marketing Inc. manufactures two products, A and B . Presently, the company uses a single plantwide factory overhead rate for allocating overhead to products. However, management is...
Buck's The Next Step Advanced Medical Coding And Auditing 1st Edition - ISBN: 0323762778 - Free Book

Study smarter with the SolutionInn App


