Fill in any unshared electrons that are missing from the following linebond structures: (a) H3C CH3 Dimethyl
Question:
Fill in any unshared electrons that are missing from the following linebond structures: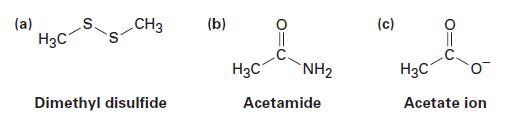
Transcribed Image Text:
(a) H3C CH3 Dimethyl disulfide (b) H3C NH₂ Acetamide (c) H3C Acetate ion
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (4 reviews)
A The given compound is Dimethylsulphide As you can see each s...View the full answer

Answered By

Samee Ullah
Algebra, Linear algebra, calculus, accounting, marketing, statistics, programming, real estate, writing, human resource management, business communication, Engineering: civil, chemical, electrical, mechanical, aerospace, building
Linguistics: sociolinguistics, applied linguistics, music, social sciences, biology, chemistry: all types, Thermodynamics, mechanics, modern physics, quantum physics, metaphysics, biology.
Feel free to contact us for all these subjects,; for quality, and best responses. Thankyou
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Fill in any unshared electron pairs that are missing from the following formulas: (a) (CH3CH2)2NH (b) (c) CH3CH2SCH2CH3 (d) CH3OCH2CH2OH CH, O=U
-
Fill in any nonbonding valence electrons that are missing from the followingstructures: CH (b) (c) (a) " NH2 - Dimethyl disulfide Acetate ion Acetamide
-
Add any missing unshared electron pairs (if any), then, using curved arrows to show the shifts in electrons, write the contributing resonance structures and resonance hybrid for each of the...
-
Under a plan of complete liquidation, Coast Corporation distributes land with a $300,000 adjusted basis and a $400,000 FMV to William, a 25% shareholder. William has a $200,000 basis in his Coast...
-
Determine whether each of the following is true or false. Assume that each organization is a private, not-for-profit entity. It is possible that an item need not be reported on the hospitals...
-
For the nozzle described in previous problem, 4-1-44[BXF], plot how exit velocity (V2) varies with isentropic efficiency of nozzle varying from 70% to 100%, all other input parameters remaining...
-
Larry Dundee is the chief executive offi cer of Palmer Electronics. Dundee is an expert engineer but a novice in accounting. Dundee asks you, as an accounting student, to explain (a) the bases for...
-
Big Arber Company ordered parts from a foreign supplier on November 20 at a price of 50,000 pijios when the spot rate was $0.20 per pijio. Delivery and payment were scheduled for December 20. On...
-
Suppose you can deposit at most $300 per month for your savings and you want to buy securities that are available only in large denominations. What benefit of mutual funds would be particularly...
-
1. Andrew Mason admits that Groupon has thousands of copycats, yet his assessment is that imitating Groupon is difficult. Do you agree? 2. Assess the bases of Groupons resources and capabilities...
-
Use the electronegativity values in Figure 1.14 to predict which of the indicated bonds in each of the following sets is more polar. Tell the direction of the polarity in each. (a) ClOCH 3 or ClOCl...
-
Amide ion, H 2 N - , is a stronger base than hydroxide ion, HO - . Which is the stronger acid, H 2 NOH (ammonia) or HO-H (water)? Explain.
-
Wozniacki and Wilcox form Jewel LLC, with each receiving a one-half interest in the capital and profits of the LLC. Wozniacki receives his one-half interest as compensation for tax planning services...
-
In 2020 the global distribution of sales in the industrial gas industry was as follows: i What is Air Liquides position on a GCI/GRI mapping? Global industrial gas industry 82 billion The 2020 global...
-
The General Social Survey polled a sample of 1048 adults in the year 2010, asking them how many hours per week they spent on the Internet. The sample mean was 9.79 with a standard deviation of 13.41....
-
An article in the Archives of Internal Medicine reported that in a sample of 244 men, 73 had elevated total cholesterol levels (more than 200 milligrams per deciliter). In a sample of 232 women, 44...
-
Explain how search can be used to solve constraint satisfaction problems, such as the eight-queens problem. What difficulties arise when such problems become extremely large (e.g., the...
-
Casse (1981) developed an exercise to encourage his students to develop their empathic skills. He asked them to listen to a recording of a dialogue between John Miller (a US project manager in...
-
The following table is similar to Table 17.2, except that it includes the earnings of Asian males and females. Does the fact that Asian males are the highest-earning group in the table affects the...
-
Read the Forecasting Supply Chain Demand Starbucks Corporation case in your text Operations and Supply Chain Management on pages 484-485, then address the four questions associated with the...
-
The principle of least motion, which states that the reaction that involves the least change in atomic positions or electronic configuration (all else being equal) is favored, has been suggested to...
-
Investigation has shown that cyclobutadiene is actually a rectangular molecule rather than a square molecule. In addition, it has been established that there are two different...
-
Explain why HBr should be used to generate the benzenediazonium salt if bromobenzene is the desired product of the Sandmeyer reaction and HCl should be used if chlorobenzene is the desired product.
-
Your firm is planning to invest in an automated packaging plant. Harburtin Industries is an all - equity firm that specializes in this business. Suppose Harburtin ' s equity beta is 0 . 8 7 , the...
-
Ned Allen opened a medical practice in Los Angeles, California, and had the following transactions during the month of January. (Click the icon to view the January transactions.) Journalize the...
-
do you need more information or are you working on this? Irene Watts and John Lyon are forming a partnership to which Watts will devote one- half time and Lyon will devote full time. They have...

Study smarter with the SolutionInn App


