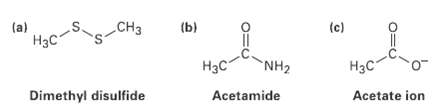
Fill in any nonbonding valence electrons that are missing from the followingstructures: CH (b) (c) (a)
Question:
Fill in any nonbonding valence electrons that are missing from the followingstructures:
Transcribed Image Text:
CHз (b) (c) (a) Нас" NH2 Нас- Нас Dimethyl disulfide Acetate ion Acetamide
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 46% (15 reviews)
a 00 H...View the full answer

Answered By

JAPHETH KOGEI
Hi there. I'm here to assist you to score the highest marks on your assignments and homework. My areas of specialisation are:
Auditing, Financial Accounting, Macroeconomics, Monetary-economics, Business-administration, Advanced-accounting, Corporate Finance, Professional-accounting-ethics, Corporate governance, Financial-risk-analysis, Financial-budgeting, Corporate-social-responsibility, Statistics, Business management, logic, Critical thinking,
So, I look forward to helping you solve your academic problem.
I enjoy teaching and tutoring university and high school students. During my free time, I also read books on motivation, leadership, comedy, emotional intelligence, critical thinking, nature, human nature, innovation, persuasion, performance, negotiations, goals, power, time management, wealth, debates, sales, and finance. Additionally, I am a panellist on an FM radio program on Sunday mornings where we discuss current affairs.
I travel three times a year either to the USA, Europe and around Africa.
As a university student in the USA, I enjoyed interacting with people from different cultures and ethnic groups. Together with friends, we travelled widely in the USA and in Europe (UK, France, Denmark, Germany, Turkey, etc).
So, I look forward to tutoring you. I believe that it will be exciting to meet them.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Fill in any unshared electron pairs that are missing from the following formulas: (a) (CH3CH2)2NH (b) (c) CH3CH2SCH2CH3 (d) CH3OCH2CH2OH CH, O=U
-
Fill in the reagents a that are missing from the followingscheme: CC -CH CH . H ,
-
Certain item descriptions and amounts are missing from the monthly schedule of cost of goods manufactured below and the income statement of Tretinik Manufacturing. Fill in the missing items. TRETINIK...
-
What are the advantages and disadvantages of seismic reflection data sets?
-
Blue Company, an architectural firm, has a bookkeeper who maintains a cash receipts and disbursements journal. At the end of the year (2016), the company hires you to convert the cash receipts and...
-
A bucket of mass m is tied to a massless cable that is wrapped around the outer rim of a frictionless uniform pulley of radius R, similar to the system shown in Fig. E9.47. In terms of the stated...
-
What does the organization seem to value?
-
The standard heat of combustion (Hc) of liquid 2.3,3-trimethylpentane [C8H18] is reported in a table of physical properties to be 4850 kJ/mol. A footnote indicates that the reference temperature for...
-
Remaining Time: 13 minutes, 11 seconds. Question Completion Status: Moving to the next question prevents changes to this answer. Question 11 Preparing closing entries by using the adjusted trail...
-
Prepare the closing entries from the following selected accounts from the records of North Pole Enterprises at December 31, 2018: How much net income did North Pole Enterprises earn during 2018?...
-
Draw a line-bond structure for vinyl chloride, C2H3C1, the starting material from which PVC [poly (vinyl chloride)] plastic is made.
-
Convert the following line-bond structures into molecularformulas: CH CH- (a) (b) . Hi H. c=C Vitamin C Aspirin (acetylsalicylic acid) (ascorbic acid) (c) H. - (d) H~ CH c-o H. . HOI I H H OH...
-
North Face is one of the world's most popular outdoor apparel companies. Assume that North Face borrows $2 million from U.S. Bank and signs a note promising to pay the $2 million back in nine months,...
-
A year-end cut-off error occurred in 2017. A large shipment of nonperishable supplies arrived from South America on the last day of 2017 and had been left in the shipping containers outside the main...
-
15. [5] It's not so difficult to incorporate time-varying volatility into the BSM model as long as the time variation is not random. Assume a BSM economy, but this time, assume that the volatility of...
-
3.6. Explain and discuss the potential benefits to be gained by using blade twist, plan- form taper, low solidity, large radius, and low rotational speed for the main rotor of a heavy lift helicopter...
-
2. A VRM (Voltage Regulator Modul) is used to supply the voltageto the CPU of a computer. In the new generation of microprocessors,whose power consumption is 100W, the input voltage to the VRM is12V...
-
Alvarado Company produced 6,400 units of product that required 5.5 standard direct labor hours per unit. The standard variable overhead cost per unit is $5.80 per direct labor hour. The actual...
-
High-low analysis, scatter chart analysis and regression analysis as applied to separating costs into fixed and variable components. LO.1
-
In Exercises 1558, find each product. (9 - 5x) 2
-
Calculate the vapor pressure of n-decane as a function of temperature using the Peng-Robinson equation of state. Compare your results with (a) Literature values (b) Predictions using the...
-
Bond strengths can be used to estimate the relative stability of isomers that have different bonds. The isomer that has the larger total bond energy is more stable. One of the following isomers is...
-
Bond strengths can be used to estimate the relative stability of isomers that have different bonds. The isomer that has the larger total bond energy is more stable. One of the following isomers is...
-
One of the isomers of C 5 H 12 reacts with Cl 2 in the presence of light to produce three isomers of C 5 H 11 Cl: This reaction replaces am one of the hydrogen?s of C 5 H 12 with a Cl. What arc the...
-
The Regal Cycle Company manufactures three types of bicyclesa dirt bike, a mountain bike, and a racing bike. Data on sales and expenses for the past quarter follow: Total Dirt Bikes Mountain Bikes...
-
?? A local college is deciding whether to conduct a campus beautification initiative that would imvolve various projects, such as planting trees and remodeling bulidings, to make the campus more...
-
A company has net income of $196,000, a profit margin of 9.7 percent, and an accounts receivable balance of $135,370. Assuming 70 percent of sales are on credit, what is the companys days sales in...

Study smarter with the SolutionInn App


