Convert the following line-bond structures into molecularformulas: CH CH- (a) (b) . Hi H. c=C
Question:
Convert the following line-bond structures into molecularformulas:
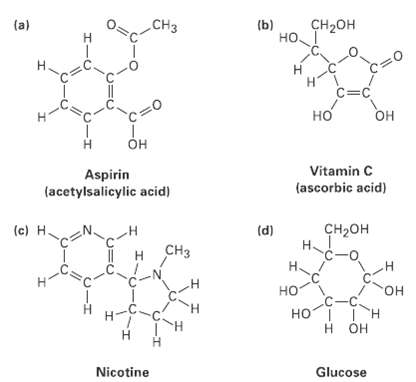
Transcribed Image Text:
CHз CH-он (a) (b) но. Hi H. c=C но Он он Vitamin C Aspirin (acetylsalicylic acid) (ascorbic acid) (c) H. сн-он (d) H~ CHз c-o H. н но но. HOI I H H OH Nicotine Glucose
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 68% (16 reviews)
In molecular formulas of organic molecules carb...View the full answer

Answered By

PALASH JHANWAR
I am a Chartered Accountant with AIR 45 in CA - IPCC. I am a Merit Holder ( B.Com ). The following is my educational details.
PLEASE ACCESS MY RESUME FROM THE FOLLOWING LINK: https://drive.google.com/file/d/1hYR1uch-ff6MRC_cDB07K6VqY9kQ3SFL/view?usp=sharing
3.80+
3+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Related Video
For this experiment, we\'ve compared the freshness of flowers by keeping them in three separate bottles filled with water, aspiring water, and food plant
Students also viewed these Organic Chemistry questions
-
Convert the following condensed structures into skeletal structures (remember that condensed structures show atoms but few, if any, bonds, whereas skeletal structures show bonds but few, if any,...
-
Convert the following hashed-wedged line formulas into condensed formulas. (a) (b) (c) in NH2 Br H-C Br Br
-
Convert the following condensed formulas into hashed-wedged line structures. (a) (b) CHCI3 (c) (CH3)2NH (d) cHuCHOCL SH ,
-
For Table 24.1, suppose you think that you omitted a relevant variable, physicians per capita. Would this harm your results for S or H? Explain your view. Table 24.1 (Model 1) The Effect of Community...
-
In 2015, Juan entered into a contract to write a book. The publisher advanced Juan $50,000, which was to be repaid out of future royalties. If the book was not completed by the end of 2016, however,...
-
An article in Optical Engineering [Operating Curve Extraction of a Correlators Filter (2004, Vol. 43, pp. 27752779)] reported on the use of an optical correlator to perform an experiment by varying...
-
Does the culture appear to be strong or weak?
-
In the current year, Madison Corporation had $50,000 of taxable income at a tax rate of 25%. During the year, Madison began offering warranties on its products and has a warranty liability for...
-
Messier Backyard Rink Co. produces a single product. The cost of producing and selling a single backyard rink at the companys normal activity level of 750 units per month is as follows: Direct...
-
INVOLVE was incorporated as a not-for-profit voluntary health and welfare organization on January 1, 2017. During the fiscal year ended December 31, 2017, the following transactions occurred. 1. A...
-
Fill in any nonbonding valence electrons that are missing from the followingstructures: CH (b) (c) (a) " NH2 - Dimethyl disulfide Acetate ion Acetamide
-
Convert the following molecular formulas into line-bond structures that are sonsistent with valence rules: (a) C3H8 (b) CH5N (c) C2H6O (2 possibilities) (d) C3H7Br (2 possibilities) (e) C2H4O (3...
-
DefineThermal efficiency and mechanical efficiency of I.C. engine.
-
A company must decide between scrapping or reworking units that do not pass inspection. The company has 16,000 defective units that have already cost $132,000 to manufacture. The units can be sold as...
-
according to the phase rule, the triple point of a pure substance is A. invariant B. u nivariant C. bivariant D. none of the above
-
33. If the equipment in the previous question had sold for $15,000, the correct entry would be: a. Cash debit $15,000. Gain credit $3,000. $12,000 Equipment credit b. Cash debit $15,000. Debit a loss...
-
The banks play a central role in financial intermediation in New Zealand. 1.What is financial intermediation? Who performs it? and why is it important? 2.What is Qualitative Asset transformation...
-
Consider the following information attributed to the material management department Budgeted usage of materials - handling labor - hours 3,700 Budgeted cost pools: Fixed costs $166,500 Variable costs...
-
The statistical properties of regression analysis such as the standard error, the variance, correlation, and their use in determining which is the best rearession and how good the best regression is....
-
Fill in each blank so that the resulting statement is true. 83 + 103 = ______ .
-
The figure below shows a pressure-enthalpy diagram submitted by Joe Udel as part of a homework assignment. On the diagram isotherms (T 1 2 3), isochores ( V 1 V 2 V 3 ), and isentropes ( S 1 S 2 S 3...
-
On reaction with Cl 2 in the presence of light, an unknown compound with the formula C 5 H 10 gives only one isomer of C 5 H 9 Cl (see problem 2.39). What is the DU of the unknown compound? Show the...
-
Explain how the dipole moment for CH3Cl ( = 1.9 D) can be larger than the dipole moment for CH3F ( = 1.8 D).
-
Peptides arc smaller versions of proteins and have similar functional groups. What functional groups are present in this peptide? OH T CH, O 1 CH3 0 I HN-CH-C-NH-CH-C-NH-CH-C-OH Serine-alanine-glycine
-
Hrubec Products, Incorporated, operates a Pulp Division that manufactures wood pulp for use in the production of various paper goods. Revenue and costs associated with a ton of pulp follow: Selling...
-
The AICPA guidelines suggest that taxes should be transparent and visible. This means that: a. The taxes affect similarly situated taxpayers in a similar manner. b. Taxes should be due at the same...
-
What is Apple Companys strategy for success in the marketplace? Does the company rely primarily on customer intimacy, operational excellence, or product leadership? What evidence supports your...

Study smarter with the SolutionInn App


