From data in Table 16.4, determine (a) K a for C 5 H 5 NH + ;
Question:
From data in Table 16.4, determine
(a) Ka for C5H5NH+;
(b) Kb for HCOO-;
(c) Kb for C6H5O-.
Table 16.4
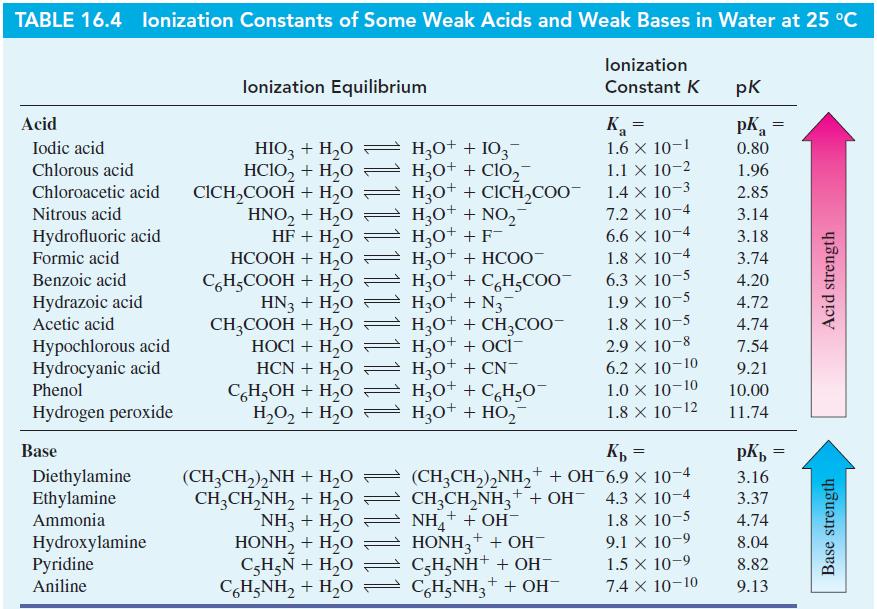
Transcribed Image Text:
TABLE 16.4 lonization Constants of Some Weak Acids and Weak Bases in Water at 25 °C lonization Constant K Acid Iodic acid Chlorous acid Chloroacetic acid Nitrous acid Hydrofluoric acid Formic acid Benzoic acid Hydrazoic acid Acetic acid Hypochlorous acid Hydrocyanic acid Phenol Hydrogen peroxide Base Diethylamine Ethylamine Ammonia Hydroxylamine Pyridine Aniline lonization Equilibrium HIO3 + H₂O → H3O+ + 103¯ HCIO₂ + H₂O H3O+ + ClO₂ CICH₂COOH + H₂O HNO₂ + H₂O HF + H₂O HCOOH + H,O C6H₂COOH + H₂O HN3 + H₂O CH3COOH + H₂O HOCI + H₂O HCN + H₂O C6H₂OH + H₂O H3O+ + CICH₂COO™ H3O+ + NO₂ H₂O+ + F- H,O+ + HCOO- H3O+ + C6H₂COO™ H₂O + N₂¯ H3O+ + CH3COO- H₂O+ + OCI- H3O+ + CN- H3O+ + C6H₂O H,O,+ H,O = H,O* + HO, H₂O+ (CH3CH₂)₂NH + H₂O CH,CH,NH, + H,O NH3 + H₂O HONH, + H,O C5H₂N + H₂O CH;NH, + H,O Ka 1.6 X 10-1 1.1 X 10-2 = 1.4 x 10-3 7.2 x 10-4 6.6 x 10-4 1.8 x 10-4 6.3 × 10-5 1.9 × 10-5 1.8 X 10-5 2.9 × 10-8 6.2 X 10-10 1.0 × 10-10 1.8 X 10-12 Kb = 1.8 x 10-5 (CH3CH₂)₂NH₂+ + OH-6.9 × 10-4 CH3CH₂NH3+ + OH- 4.3 × 10-4 NH₂+ + OH- HONH3 + + OH- C-H5NH+ + OH- C6H5NH₂+ + OH- 9.1 x 10-9 1.5 × 10-9 7.4 X 10-10 pk pk = a 0.80 1.96 2.85 3.14 3.18 3.74 4.20 4.72 4.74 7.54 9.21 10.00 11.74 pKb 3.16 3.37 4.74 8.04 8.82 9.13 = Acid strength Base strength
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (6 reviews)
a Ka for CHNH is 666x106 b Kb for HCOO is ...View the full answer

Answered By

CHARLES AMBILA
I am an experienced tutor with more than 7 years of experience. I have helped thousands of students pursue their academic goals. My primary objective as a tutor is to ensure that students have easy time handling their academic tasks.
5.00+
109+ Reviews
324+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
Determine the constant C in Eq. 40-27 to five significant figures by finding C in terms of the fundamental constants in Eq. 40-24 and then using data from Appendix B to evaluate those constants....
-
Table 11.11 is from a Kansas State University survey of 262 pig farmers. For the question What are your primary sources of veterinary information?, the categories were (A) professional consultant,...
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-6. On December 12, Irene purchased the building where her store is located. She paid...
-
Explain the difference between impregnation and infiltration. Give some applications for each?
-
JLB Corporation is attempting to determine whether to lease or purchase research equipment. The firm is in the 40% tax bracket, and its after tax cost of debt is currently 8%. The terms of the lease...
-
Educational testing companies provide tutoring, classroom learning, and practice tests in an effort to help students perform better on tests such as the Scholastic Aptitude Test (SAT). The test...
-
2. Exhibit 11.16 presents the income statement and balance sheet for PartsCo, a $1.2 billion supplier of machinery parts. The company is expected to increase revenues by 8 percent annually for the...
-
Ray County administers a tax custodial fund, an investment trust fund, and a private-purpose trust fund. The tax custodial fund acts as custodian for the county, a city within the county, and the...
-
Scott Company compr mercancas con un precio de factura de $3000 y condiciones de crdito de 1/10, n/30. Suponiendo un ao de 360 das, cul es la tasa de inters anual implcita inherente a los trminos del...
-
Codeine, C 18 H 21 O 3 N, is an opiate, has analgesic and antidiarrheal properties, and is widely used. In water, codeine is a weak base. A handbook gives pK a = 6.05 for protonated codeine, C 18 H...
-
The antimalarial drug quinine, C 20 H 24 O 2 N 2 , is a diprotic base with a water solubility of 1.00 g/1900 mL of solution. (a) Write equations for the ionization equilibria corresponding to pK b1 =...
-
Two well- known models of firm value are the dividend discount model and the discounted cash flow model. Under ideal conditions, each model gives the same result. In Example 2.2, assume that P. V....
-
A popular theory is that presidential candidates have an advantage if they are taller than their main opponents. Listed are heights (in centimeters) of randomly selected presidents along with the...
-
Cash Flows Horiz Analysis Horiz Analysis Vertic Analysis Vertic Analysis from Oper Inc St Bal St Inc St Bal Sheet Ratios Requirement Prepare the cash flows from operations section of R. Ashburn...
-
Rudy Gandolfi owns and operates Rudy's Furniture Emporium Incorporated. The balance sheet totals for assets, liabilities, and stockholders' equity at August 1, 2022, are as indicated. Described here...
-
The brand manager for a brand of toothpaste must plan a campaign designed to increase brand recognition. He wants to first determine the percentage of adults who have heard of the brand. How many...
-
Pulse rates of women are normally distributed with a mean of 77.5 beats per minute and a standard deviation of 11.6 beats per minute. Answer the following questions. What are the values of the mean...
-
Cabo Company has $1,000,000 in assets and $1,000,000 in stockholders' equity, with 40,000 shares outstanding the entire year. It has a return on assets of 10%. During 2016, it had net income of...
-
PC Contractors, Inc., was an excavating business in Kansas City, Missouri. Union Bank made loans to PC, subject to a perfected security interest in its equipment and other assets, including...
-
The amounts of the assets and liabilities of Heavenly Travel Service at April 30, 2010, the end of the current year, and its revenue and expenses for the year are listed below. The capital of...
-
Doug Van Buren established Ohm Computer Services on July 1, 2010. The effect of each transaction and the balances after each transaction for July are shown at the top of the following page....
-
The financial statements at the end of Four Corners Realty's first month of operations are shown below and on the shown below. Instructions By analyzing the interrelationships among the four...
-
Slow Roll Drum Co. is evaluating the extension of credit to a new group of customers. Although these customers will provide $198,000 in additional credit sales, 13 percent are likely to be...
-
Wendell's Donut Shoppe is investigating the purchase of a new $39,600 conut-making machine. The new machine would permit the company to reduce the amount of part-time help needed, at a cost savings...
-
1.Discuss the challenges faced with Valuing Stocks and Bonds. As part of this discussion, how will the selected item be implemented in an organization and its significance? 2. Discuss how Valuing...

Study smarter with the SolutionInn App


