From Figure 12-18, estimate (a) The vapor pressure of C 6 H 5 NH 2 at 100C
Question:
From Figure 12-18, estimate
(a) The vapor pressure of C6H5NH2 at 100°C
(b) The normal boiling point of C6H5CH3.
Figure 12-18
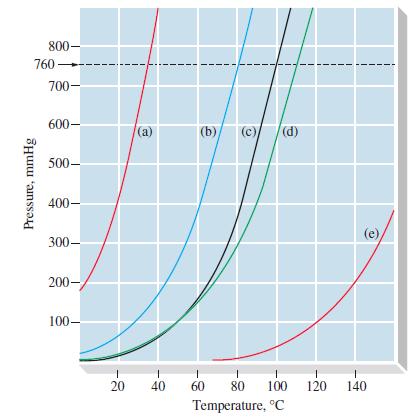
Transcribed Image Text:
800- 760- Pressure, mmHg 700- 600- 500- 400- 300- 200- 100- 20 (a) (b) (c) (d) 1 1 1 40 60 80 100 Temperature, °C 1 120 1 140
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
a It appears that the vapor pressure of C6H5NH2 ...View the full answer

Answered By

Collins Omondi
I have been an academic and content writer for at least 6 years, working on different academic fields including accounting, political science, technology, law, and nursing in addition to those earlier listed under my education background.
I have a Bachelor’s degree in Commerce (Accounting option), and vast knowledge in various academic fields Finance, Economics, Marketing, Management, Social Science, Women and Gender, Business law, and Statistics among others.
4.80+
4+ Reviews
16+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
Procurement of commodities is a critical activity for a company's survival, particularly in the manufacturing industry. It is essential in the industry because it standardizes the procurement of...
-
The normal boiling point for acetone is 56.5oC. At an elevation of 5300 ft, the atmospheric pressure is 630 torr. What would be the boiling point of acetone (Hvap = 32.0 kJ/mol) at this elevation?...
-
The vapor pressure of liquid benzene is 20,170 Pa at 298.15 K, and Î H vaporization =30.72 kJ mol -1 at 1 atm pressure. Calculate the normal and standard boiling points. Does your result for...
-
Goddard Company has used the FIFO method of inventory valuation since it began operations in 2015. Goddard decided to change to the average cost method for determining inventory costs at the...
-
Fred Lane, who sells boats, motors, and trailers, sold a boat, motor, and trailer to John Willis in exchange for a check for $6,285.00. The check was not honored when Lane attempted to use the funds....
-
The financial statements for Apple Inc. are presented in Appendix A. Instructions Answer these questions using the Statement of Operations. a. What was the percentage change in net sales and in net...
-
Why is sanitation such an important issue in foodservice operations?
-
The auditor of Cubs obtained the following client-prepared bank reconciliation: The auditor for Cubs Co. has obtained the client-prepared bank reconciliation. The following information is available: ...
-
LEIFLI D E G H M N Requirements 1 Complete the data table. Enter all amounts as positive values. Do not use a minus sign or parentheses for any values. 2 Using the present value of an ordinary...
-
Bill and Kate Theil are not only husband and wife but entrepreneurs who have established three successful businesses. The proposed plan for their latest effort involves a series of international...
-
Use data in Figure 12-20 to estimate (a) The normal boiling point of aniline; (b) The vapor pressure of diethyl ether at 25 C. Figure 12-20 P 6.75 - 6.50- 6.25 6.00- 5.75- 5.50- 5.25 5.00 4.75 - 4.50...
-
A 50.0 g piece of iron at 152 C is dropped into 20.0 g H 2 O(l) at 89 C in an open, thermally insulatedcontainer. How much water would you expect to vaporize, assuming no water splashes out? The...
-
Which of the following are required for a malicious prosecution case? a. The defendant must actively participate in instigating the prosecution. b. The proceedings must have been in the defendants...
-
Use your understanding of work and power to answer the following questions. 1. Two physics students, Will N. Andable and Ben Pumpiniron, are in the weightlifting room. Will lifts the 100-pound...
-
Problem 2. Consider the following chemical reaction. 2H2 + O2 = 2HO Gibbs Duhem equation states that SdT - Vdp+ Nidi=0. Apply this equation for the above reaction and determine the equilibrium...
-
Part D: Exploring Pascal's Triangle 1. Fill-In the missing numbers in Pascal's Triangle. See 2. Find the sum of each row in Pascal's Triangle. Describe the pattern. 1, 2, 4, 8, 16... Power of 2n 1 1...
-
A new partner C is invited to join in the AB partnership. Currently, A's and B's capital are $540,000 and $100,000, respectively. According to their profit and loss sharing contract, partner A and B...
-
The two tanks shown are connect through a mercury manometer. What is the relation between ???? and ? water Az water Ah
-
Write two resonance structures for the benzoate ion (C6H5CO2-) that show how the negative charge is delocalized over the two oxygens. Can the negative charge in the benzoate ion be delocalized into...
-
Reduction in sales All of the above 29. Belt of an electric motor is broken, it needs a. Corrective maintenance b. Scheduled maintenance c. Preventive maintenance d. Timely maintenance. 30. The...
-
Capital Gains versus Income Consider four different stocks, all of which have a required return of 18 percent and a most recent dividend of $4.50 per share. Stocks W, X, and Y are expected to...
-
Stock Valuation most corporations pay quarterly dividends on their common stock rather than annual dividends. Barring any unusual circumstances during the year, the board raises, lowers, or maintains...
-
Non constant Growth Storico Co. just paid a dividend of $2.75 per share. The company will increase its dividend by 20 percent next year and will then reduce its dividen4 growth rate by 5 percentage...
-
Winter Time Adventures is going to annual dividend if $2.61 a share on its common stock next week. This year, the company paid a dividend of $2.50 a share. The company adheres to a constant rate of...
-
Small Factory : -Regular time 8 hours per day. -1 hour daily lunch break. -25 working days per month. -50 workers. -Worker productivity 2.5 units per hour. -sold for $ 150 per unit. -cost of Labor...
-
$500 is invested for 7 years at 10 % p.a. simple interest. How much will the investment be worth after this period

Study smarter with the SolutionInn App


