In a heat engine, heat (q h ) is absorbed by a working substance (such as water)
Question:
In a heat engine, heat (qh) is absorbed by a working substance (such as water) at a high temperature (Th). Part of this heat is converted to work (w), and the rest (q1) is released to the surroundings at the lower temperature (T1) The efficiency of a heat engine is the ratio w/qh. The second law of thermodynamics establishes the following equation for the maximum efficiency of a heat engine, expressed on a percentage basis.
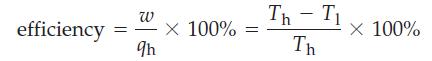
In a particular electric power plant, the steam leaving a steam turbine is condensed to liquid water at 41 °C (T1) and the water is returned to the boiler to be regenerated as steam. If the system operates at 36% efficiency,
(a) What is the minimum temperature of the steam [H2O(g)] used in the plant?
(b) Why is the actual steam temperature probably higher than that calculated in part (a)?
(c) Assume that at Th the H2O(g) is in equilibrium with H2O(l). Estimate the steam pressure at the temperature calculated in part (a).
(d) Is it possible to devise a heat engine with greater than 100 percent efficiency? With 100 percent efficiency? Explain.
Step by Step Answer:

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette





