It is possible to write simple equations to relate pH, pK, and concentrations (c) of various solutions.
Question:
It is possible to write simple equations to relate pH, pK, and concentrations (c) of various solutions. Three such equations are shown here.
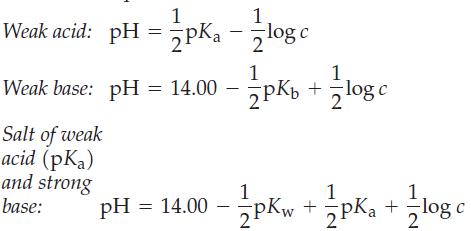
(a) Derive these three equations, and point out the assumptions involved in the derivations.
(b) Use these equations to determine the pH of 0.10 M CH3COOH(aq), 0.10 M NH3(aq), and 0.10 M NaCH3COO(aq).
Verify that the equations give correct results by determining these pH values in the usual way.
Transcribed Image Text:
1 Weak acid: pH = pK₁ - 1log c 1 Weak base: pH = 14.00 --- pKb 2PK₂ Salt of weak acid (pka) and strong base: +=log c 1 1 pH = 14.00 - 2pKw+ Pka + log c
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
Lets derive and discuss the three given equations for weak acids weak bases and salts of weak acids and strong bases After that well use these equatio...View the full answer

Answered By

Asim farooq
I have done MS finance and expertise in the field of Accounting, finance, cost accounting, security analysis and portfolio management and management, MS office is at my fingertips, I want my client to take advantage of my practical knowledge. I have been mentoring my client on a freelancer website from last two years, Currently I am working in Telecom company as a financial analyst and before that working as an accountant with Pepsi for one year. I also join a nonprofit organization as a finance assistant to my job duties are making payment to client after tax calculation, I have started my professional career from teaching I was teaching to a master's level student for two years in the evening.
My Expert Service
Financial accounting, Financial management, Cost accounting, Human resource management, Business communication and report writing. Financial accounting : • Journal entries • Financial statements including balance sheet, Profit & Loss account, Cash flow statement • Adjustment entries • Ratio analysis • Accounting concepts • Single entry accounting • Double entry accounting • Bills of exchange • Bank reconciliation statements Cost accounting : • Budgeting • Job order costing • Process costing • Cost of goods sold Financial management : • Capital budgeting • Net Present Value (NPV) • Internal Rate of Return (IRR) • Payback period • Discounted cash flows • Financial analysis • Capital assets pricing model • Simple interest, Compound interest & annuities
4.40+
65+ Reviews
86+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
Do some amendment and enhance the given research paper: Table of Content Abstract..3 Action Research.4 Research Methodology and Design...5 Literature Review: NoSQL Database7 Proposal.7 Iteration 1..8...
-
A sample containing an alkali sulfate is dried, weighed and dissolved in dilute HCl. Barium chloride solution is added in excess to precipitate barium sulfate, and the precipitate is digested in the...
-
In February 19_8, Randy White, president of Arriscraft Corporation (Arriscraft), had just received two requests for a price on two of their marble products. The first request was from a nearby city...
-
Alpha Appliance Service had net income for the year of $ 35,000. In addition, the balance sheet reports the following balances: Calculate the return on assets (ROA) for Alpha Appliance Service for...
-
Using the information given in Exercise 1626 and assuming pretax financial income of $3,100,000, calculate taxable income.
-
The local amateur football club spent $675 on tickets to a professional football game. If the club bought three fewer 15-dollar tickets than four-fifths the number of 12-dollar tickets, how many...
-
a. Prepare the journal entry to record Tamasine Companys issuance of 5,000 shares of $100 par value 7% cumulative preferred stock for $102 cash per share. b. Assuming the facts in part 1, if Tamasine...
-
The Coca-Cola Companys manufacturing operations are ideal for process costing because it and its bottlers produce long runs of identical beverages in a continuous flow production process. Go to the...
-
Future Stars Preschool daycare operates a not-for-profit morning daycare that operates nine months of the year. Future Stars has 161 kids enrolled in its various programs. Future Star's primary...
-
For the ionization of phenylacetic acid, CHCHCOH + HO H3O+ + CH5CHCO Ka 4.9 x 10-5 = (a) What is [C6H5CHCO2] in 0.186 M C6H5CHCOH? (b) What is the pH of 0.121 M C6H5CHCOH?
-
For H 2 SO 3 (aq), K a1 = 1.3 x 10 -2 and K a2 = 6.3 x 10 -8 . In 0.10 M H 2 SO 3 (aq), (a) [HSO3] = 0.013 M; (b) [SO3] = 6.3 108 M; (c) [H3O] = 0.10 M; (d) [H3O] = 0.013 M; (e) [SO3-] = 0.036 M.
-
The Fourier coefficients are to be found numerically when the values of the function \(f(t)\) are available a. in analytical form b. at discrete values of \(t\) c. in the form of a complex equation
-
Lifestyle is how one enacts the self-concept. The way they would enact it is through buying luxury items which is the most premium iPhone. The latent reasons why people want an iPhone 15 all have to...
-
Make a Tows Matrix that assess the strengths, weakness, opportunities, and threats for Dannon based on the case study For typical corporate strategies under purpose of communication. Strengths 1) 2)...
-
Now that you've watched the lectures, The Abilene Paradox movie, and the Challenger Disaster Video, I'd like you to think for a moment about when you may have observed the Abilene Paradox or...
-
Ensuring that the projectadheres to the selected quality standard . Often, ensuring that the project work is done 'correctly' is as important as ensuring that the end result fulfills the project's...
-
Think about some career planning and development issues; for example, mergers and reorganization uncertainty, lack of upward mobility, getting managers to understand your career potential, and...
-
We note from the exponents in Eq. (8.30) that the cutting speed has a greater influence on temperature than does the feed. Why?
-
In the simple quantity theory of money, what will lead to an increase in aggregate demand? In monetarism, what will lead to an increase in aggregate demand?
-
J. Lynn, M. Oller, and F. Tate share income on a 5 : 3 : 2 basis. They have capital balances of $30,000, $26,000, and $18,000, respectively, when Doc Duran is admitted to the partnership....
-
G. Olde and R. Young share income on a 6 : 4 basis. They have capital balances of $100,000 and $70,000, respectively, when K.Twener is admitted to the partnership. Instructions Prepare the journal...
-
B. Cates, V. Elder, and S. Nguyen have capital balances of $50,000, $40,000, and $32,000, respectively. Their income ratios are 5 : 3 : 2. Nguyen withdraws from the partnership under each of the...
-
If you purchase a $1000 par value bond for $1065 that has a 6 3/8% coupon rate and 15 years until maturity, what will be your annual return? 5.5% 5.9% 5.7% 6.1%
-
Famas Llamas has a weighted average cost of capital of 8.8 percent. The companys cost of equity is 12 percent, and its pretax cost of debt is 6.8 percent. The tax rate is 22 percent. What is the...
-
The common stock of a company paid 1.32 in dividens last year. Dividens are expected to gros at an 8 percent annual rate for an indefinite number of years. A) If the company's current market price is...

Study smarter with the SolutionInn App


