Lead metal is added to 0.100 M Cr 3+ (aq). What are [Pb 2+ ], [Cr 2+
Question:
Lead metal is added to 0.100 M Cr3+(aq). What are [Pb2+], [Cr2+], and [Cr3+] when equilibrium is established in the reaction?
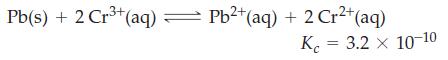
Transcribed Image Text:
3+ Pb(s) + 2 Cr³+ (aq) = Pb²+ (aq) + 2 Cr²+ (aq) K 3.2 x 10-10 =
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
The reaction is as follows Pbs 2 Cr3aq Pb2aq 2 Cr2aq The equilibrium constant is Kc 32 ...View the full answer

Answered By

Joseph Mwaura
I have been teaching college students in various subjects for 9 years now. Besides, I have been tutoring online with several tutoring companies from 2010 to date. The 9 years of experience as a tutor has enabled me to develop multiple tutoring skills and see thousands of students excel in their education and in life after school which gives me much pleasure. I have assisted students in essay writing and in doing academic research and this has helped me be well versed with the various writing styles such as APA, MLA, Chicago/ Turabian, Harvard. I am always ready to handle work at any hour and in any way as students specify. In my tutoring journey, excellence has always been my guiding standard.
4.00+
1+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
When magnesium metal is added to a beaker of HCl(aq), a gas is produced. Knowing that magnesium is oxidized and that hydrogen is reduced, write the balanced equation for the reaction. How many...
-
An excess of zinc metal is added to 50.0 mL of a 0.100 M AgNO3 solution in a constant-pressure calorimeter like the one pictured in Figure 6.9. As a result of the reaction The temperature rises from...
-
Let us explore a reaction with a limiting reactant. Here, zinc metal is added to a flask containing aqueous HCl, and H 2 gas is a product. The three flasks each contain 0.100 mol of HCl. Zinc is...
-
Herbs Pty Ltd is considering investing in a new herb packaging machine. The machine is estimated to cost $80,000 which can last for 7 years before it becomes too costly to maintain and can be sold...
-
Wells and Associates has EBIT of $67,500. Interest costs are $22,500, and the firm has 15,000 shares of common stock outstanding. Assume a 40% tax rate. a. Use the degree of financial leverage (DFL)...
-
Point for Discussion: In this case should the criminal prosecution for the tickets be included in the case by Nathan against Adam?
-
2. Identify the value drivers embedded in the equity cash flow model. How do the equity cash flow drivers differ from the drivers of the enterprise DCF models?
-
Create a class named Pizza with data fields for description (such as sausage and onion) and price. Include a constructor that requires arguments for both fields and a method to display the data....
-
ant 116 Second Evaluation Chapter 12 am Valuation Chapter 12 and 13(First Part Take Hom 13. Barton and Fallows form a part accounts receivable with a face amount depreciation of $100,000. The...
-
One sketch below represents an initial nonequilibrium mixture in the reversible reaction Which of the other three sketches best represents an equilibrium mixture? Explain. Kc = 4.0 (8) (8) + (8)
-
One sketch below represents an initial nonequilibrium mixture in the reversible reaction Which of the other three sketches best represents an equilibrium mixture? Explain. 2 NO(g) + Br(g) = 2 NOBr(g)...
-
Define an annuity in general terms. Describe the cash flows related to an annuity from the viewpoint of the lender in terms of receipts and payments.
-
Are investors too impatient in wanting returns from these investments? "Would Nordstrom be better off if it were owned by private investors, rather than as a publicly traded firm since this would...
-
how AI is used to discover new material, will it change the whole science environment?
-
QUESTION 1 Which one of the following statements is not part of the "bundle of rights" enjoyed by a fee simple owner of property? a. The right to possess the property b. The right to control what...
-
In order to pay his rent, Tom, a college student, has taken a job in the computer department of a local department store. His only responsibility is to answer telephone calls to the department, most...
-
How does database normalization impact data integrity and query performance in complex relational schemas ? Explain
-
Emilio and Rene Santos own Club Fandango. From its inception, Club Fandango has sold merchandise on either a cash or credit basis, but no credit cards have been accepted. During the past several...
-
The executor of Gina Purcells estate has recorded the following information: Assets discovered at death (at fair value): Cash . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . ....
-
McLean Company produces a product that requires three standard gallons per unit. The standard price is $18.50 per gallon. If 2,500 units required 8,000 gallons, which were purchased at $18.00 per...
-
Norris Company produces a product that requires 3.5 standard hours per unit at a standard hourly rate of $12 per hour. If 500 units required 1,500 hours at an hourly rate of $11.50 per hour, what is...
-
McLean Company produces a product that requires two standard hours per unit at a standard hourly rate of $18 per hour. If 2,500 units required 5,500 hours at an hourly rate of $19 per hour, what is...
-
Minden Company introduced a new product last year for which it is trying to find an optimal selling price. Marketing studies suggest that the company can increase sales by 5,000 units for each $2...
-
Prepare the adjusting journal entries and Post the adjusting journal entries to the T-accounts and adjust the trial balance. Dresser paid the interest due on the Bonds Payable on January 1. Dresser...
-
Venneman Company produces a product that requires 7 standard pounds per unit. The standard price is $11.50 per pound. If 3,900 units required 28,400 pounds, which were purchased at $10.92 per pound,...

Study smarter with the SolutionInn App


