One glucose molecule, C 6 H 12 O 6 (s), is converted to two lactic acid molecules,
Question:
One glucose molecule, C6H12O6(s), is converted to two lactic acid molecules, CH3CH(OH)COOH(s) during glycolysis. Given the combustion reactions of glucose and lactic acid, determine the standard enthalpy for glycolysis.
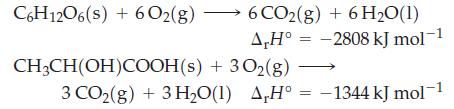
Transcribed Image Text:
C6H12O6(s) + 602(g) 6 CO2(g) + 6H₂O(1) A,H° ΔΗ° CH₂CH(OH)COOH(s) + 3O₂(g) 3CO2(g) +3H,O(1) AH° -2808 kJ mol-1 -1344 kJ mol-1
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (3 reviews)
To determine the standard enthalpy for glycolysis using the given information we can use Hesss Law H...View the full answer

Answered By

Benish Ahmad
I'm a professional software engineer. I'm lectutrer at GCUF and I have 3 years of teaching experience. I'm looking forward to getting mostly computer science work including:
Programming fundamentals
Object oriented programming
Data structures
object oriented design and analysis
Database system
Computer networks
Discrete mathematics
Web application
I am expert in different computer languages such as C++, java, JavaScript, Sql, CSS, Python and C#. I'm also have excellent knowledge of essay writing and research. I have worked in other Freelancing website such as Fiverr and Upwork. Now I have finally decided to join the SolutionInn platform to continue with my explicit work of helping dear clients and students to achieve their academic dreams. I deliver plagiarism free work and exceptional projects on time. I am capable of working under high pressure.
5.00+
2+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
In biological cells that have a plentiful supply of oxygen, glucose is oxidized completely to CO 2 and H 2 O by a process called aerobic oxidation. Muscle cells may be deprived of O 2 during vigorous...
-
Choose a conflict situation you experienced in a work setting. Make sure that your illustration is work-related and not personal. Prepare a formal report, with recommendations you would make, based...
-
Can we use servomotor for position control? Support the answer with necessary details
-
Consider the hydrogen atom, and assume that the proton, instead of being a point- source of the Coulomb field, is uniformly charged sphere of radius R ( < < ao), so that the Coulomb potential is now...
-
Assume that you must make a presentation to the marketing staff explaining the difference between product and period costs. Your supervisor tells you the marketing staff would also like clarification...
-
The following table shows the number of days of labor required to produce 1 unit of output of computers and wheat in France and Germany: (a) Calculate the autarky price ratios. (b) Which country has...
-
NPV Your division is considering two projects with the following cash flows (in millions): 0 2 $17 $6 $10 $9 $5 $10 2$25 2$20 Project A Project B 1 3 a. What are the projects NPVs assuming the WACC...
-
Norman Rentals can purchase a van that costs $45,000; it has an expected useful life of three years and no salvage value . Norman uses straight-line depreciation. Expected revenue is$25,000 per year....
-
andwee is not 41! please help (Last Word) For each dollar paid in taxes, approximately how much do households in the top quintile receive back in the form of government expenditures? Multiple Choice...
-
The steel shaft, 3 ft long and 4 in. in diameter, carries the end torque of 15 kip ft. Determine (a) the maximum shear stress in the shaft; and (b) the angle of twist of the shaft. Use G = 12 x 106...
-
Use standard enthalpies of formation from Table 7.2 and equation (7.22) to determine the standard enthalpy of reaction in the following reactions. Table 7.2 Eq. 7.22 (a) C3H8(g) + H(g) CH6(g) +...
-
The standard heats of combustion ( r H) of buta-1,3-diene, C 4 H 6 (g); butane, C 4 H 10 (g); and H 2 (g) are -2540.2, -2877.6, and -285.8 kJ mol-1, respectively. Use these data to calculate the heat...
-
Arthur Wesson, an unmarried individual who is age 68, reports 2013 taxable income of $160,000. He records AMT positive adjustments of $40,000 and tax preferences of $35,000. a. What is Arthur's AMT?...
-
Mod Clothiers makes women's clothes. It costs $28,000 to produce 5,000 pairs of polka-dot polyester pants. They have been unable to sell the pants at their usual price of $50.00. The company is...
-
In a mid-sized manufacturing company, the annual financial statements were prepared for audit by an external auditing firm. The company\'s finance team had diligently compiled the financial data, and...
-
Explain the meaning of the SMART acronym. In 100-200 words, define what the words "goal" and "success" mean to you. Summarize your thoughts on whether or not the SMART model can help you become a...
-
Small town Diners has a policy of treating dividends as a passive residual. It forecasts that net earnings after taxes in the coming year will be $500,000. The firm has earned the same $500,000 for...
-
Part 1-Chi-Square Goodness-of-Fit Tests A health psychologist was interested in women's workout preferences. Of the 56 participants surveyed, 22 preferred running, 8 preferred swimming, 15 preferred...
-
What is meant by the statement "Tool life is a random variable"?
-
Show that, given a maximum flow in a network with m edges, a minimum cut of N can be computed in O(m) time.
-
Distinguish between counterbalancing and non-counterbalancing errors. Give an example of each.
-
Discuss and illustrate how a correction of an error in previously issued financial statements should be handled.
-
Prior to 2010, Heberling Inc. excluded manufacturing overhead costs from work in process and finished goods inventory. These costs have been expensed as incurred. In 2010, the company decided to...
-
Jimmy earned $85,000 in salary from his job as an engineer. He earned $3,000 in interest income. He made RPP contributions of $2,119 and contributed $15,000 to his RRSP. Given the following schedule...
-
Question 3 A . Bonika Sdn Bhd ( BSB ) manufactures small camping tents. BSB produced 3 , 0 0 0 units during the year. These camping tents sell for RM 1 5 0 each. BSB had 5 0 0 units in finished goods...
-
Required information (The following information applies to the questions displayed below.) Davis Stores sells clothing in 15 stores located around the southwestern United States. The managers at...

Study smarter with the SolutionInn App


