Question: Ozone is a powerful oxidizing agent. Using ozone as the oxidant, write equations to represent oxidation of (a) Br (aq) to BrO (aq)
Ozone is a powerful oxidizing agent. Using ozone as the oxidant, write equations to represent oxidation of
(a) Br–(aq) to BrO–(aq) (hypobromite);
(b) NO(g) to NO2(g);
(c) Fe2+(aq) to Fe3+(aq) in acidic solution;
(d) Ag(s) to AgO(s);
(e) [Fe(CN)6]4– to [Fe(CN)6]3– in basic solution. The half-equations for the reduction of O3 in acidic and basic solutions are given in Table D.4 of Appendix D.
Table D.4
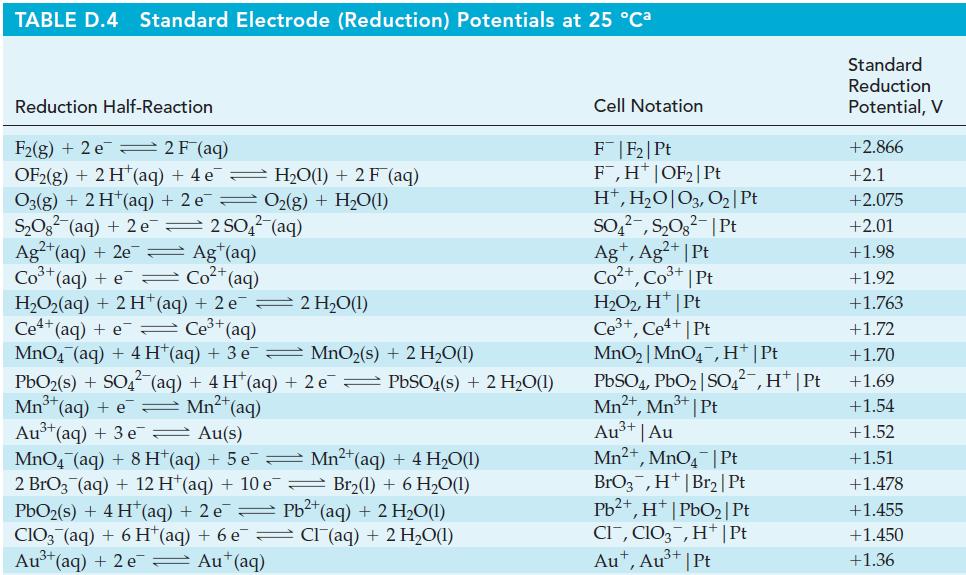
TABLE D.4 Standard Electrode (Reduction) Potentials at 25 Ca Reduction Half-Reaction F2(g) + 2 e 2 F (aq) OF2(g) + 2 H (aq) + 4e O3(g) + 2 H(aq) + 2 e H2O(1) + 2F (aq) O(g) + HO(1) SO(aq) +2e= 2 SO4-(aq) Ag(aq) + 2e Ag(aq) Co (aq) +e Co+ (aq) H,O2(aq) + 2H*(aq)+2e
Step by Step Solution
3.28 Rating (154 Votes )
There are 3 Steps involved in it
a Braq to BrOaq hypobromite Balanced equation Braq O3g H2Ol BrOaq 2Haq O2g Halfreactions O3... View full answer

Get step-by-step solutions from verified subject matter experts


