Refer to the periodic table on the inside front cover and indicate (a) The most nonmetallic element;
Question:
Refer to the periodic table on the inside front cover and indicate
(a) The most nonmetallic element;
(b) The transition metal with lowest atomic number;
(c) A metalloid whose atomic number is exactly midway between those of two noble gas elements.
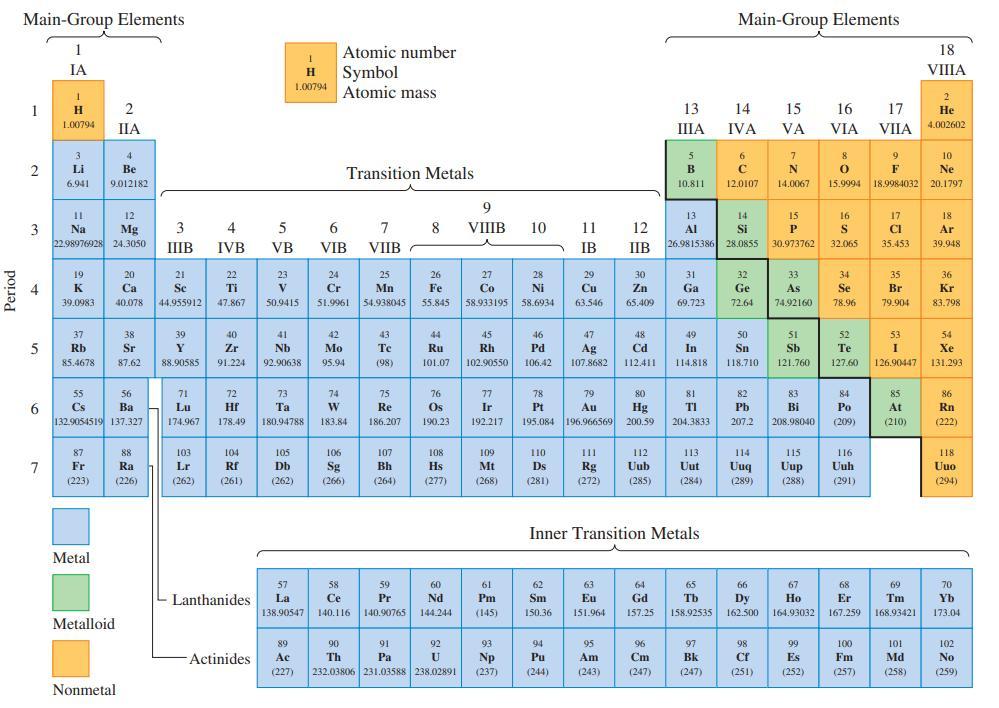
Transcribed Image Text:
Period Main-Group Elements 1 2 4 5 11 3 Na 6 IA 7 1 H 1.00794 3 Li 6.941 19 K 39.0983 12 Mg 22.98976928 24.3050 37 Rb 85.4678 55 Cs 87 Fr (223) 4 Be 9.012182 Metal 2 IIA 56 Ba 132.9054519 137.327 Metalloid 20 Ca 40.078 38 Sr 87.62 Nonmetal 88 Ra (226) 3 IIIB 21 Se 44.955912 39 Y 88.90585 71 Lu 174.967 103 Lr (262) 4 IVB 22 Ti 47.867 40 Zr 91.224 72 Hf 178.49 104 Rf (261) Lanthanides Actinides 5 VB 23 V 50.9415 1 H Symbol 1.00794 Atomic mass 41 Nb 92.90638 73 Ta 180,94788 105 Db (262) Atomic number 6 VIB Transition Metals 24 Cr 51.9961 42 Mo 95.94 74 W 183.84 106 Sg (266) 57 58 La Ce 138.90547 140.116 7 8 VIIB 25 Mn 54.938045 43 Te (98) 75 Re 186.207 107 Bh (264) 59 Pr 140.90765 26 Fe 55.845 91 Pa 44 Ru 101.07 76 Os 190.23 108 Hs (277) 60 Nd 144.244 92 89 Ac (227) 232.03806 231.03588 238.02891 90 Th U 9 VIIIB 27 Co 58.933195 45 Rh 102.90550 77 Ir 192.217 109 Mt (268) 61 Pm (145) 93 Np (237) 10 28 Ni 58.6934 46 Pd 106.42 110 Ds (281) 11 12 IB IIB 78 79 Pt Au 195.084 196.966569 62 Sm 150.36 29 Cu 63.546 94 Pu (244) 47 Ag 107.8682 30 Zn 65.409 63 Eu 151.964 48 Cd 112.411 111 112 Rg Uub (272) (285) 95 Am (243) 80 Hg 200.59 64 Gd 157.25 13 IIIA 96 Cm (247) 5 B 10.811 Inner Transition Metals 31 Ga 69.723 49 In 114.818 13 14 Al Si 26.9815386 28.0855 81 TI 204.3833 113 Uut (284) 65 Tb 158.92535 Main-Group Elements 97 Bk (247) 14 IVA 6 с 12.0107 32 Ge 72.64 50 Sn 118.710 82 Pb 207.2 114 Uuq (289) 66 Dy 162.500 98 Cf (251) 15 VA 7 N 14.0067 15 P 30.973762 33 As 74.92160 51 Sb 121.760 83 Bi 208.98040 115 Uup (288) 67 Ho 164.93032 99 Es (252) 16 VIA 8 9 0 F 15.9994 18.9984032 16 S 32.065 34 Se 78.96 52 Te 127.60 84 Po (209) 116 Uuh (291) 68 Er 167.259 17 VIIA 100 Fm (257) 17 CI 35.453 35 Br 79.904 53 I 126.90447 85 At (210) 69 Tm 168.93421 101 Md (258) 18 VIIIA 2 He 4.002602 10 Ne 20.1797 18 Ar 39.948 36 Kr 83.798 54 Xe 131.293 86 Rn (222) 118 Uuo (294) 70 Yb 173.04 102 No (259)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (1 review)
a The most non metallic element in the periodic table is F Fluorine is the ...View the full answer

Answered By

Mary Boke
As an online tutor with over seven years of experience and a PhD in Education, I have had the opportunity to work with a wide range of students from diverse backgrounds. My experience in education has allowed me to develop a deep understanding of how students learn and the various approaches that can be used to facilitate their learning. I believe in creating a positive and inclusive learning environment that encourages students to ask questions and engage with the material. I work closely with my students to understand their individual learning styles, strengths, and challenges to tailor my approach accordingly. I also place a strong emphasis on building strong relationships with my students, which fosters trust and creates a supportive learning environment. Overall, my goal as an online tutor is to help students achieve their academic goals and develop a lifelong love of learning. I believe that education is a transformative experience that has the power to change lives, and I am committed to helping my students realize their full potential.
5.00+
4+ Reviews
21+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
Refer only to the periodic table on the inside front cover and indicate which of the atoms, Bi, S, Ba, As, and Ca, (a) Is most metallic; (b) Is most nonmetallic; (c) Has the intermediate value when...
-
Refer only to the periodic table on the inside front cover, and arrange the following ionization energies in order of increasing value: the first ionization energy of F; the second ionization energy...
-
Refer to the periodic table (Figure 2.15 or inside front cover) and answer the following questions. a. What Group VIA element is a metalloid? b. What is the Group III A element in Period 3? Figure...
-
A partially completed flowchart showing some of the major documents commonly used in the purchasing function of a merchandise business is presented below. Identify documents 1, 3, and4. Purchase Order
-
Discuss the risks confronting an interest rate and currency swap dealer.
-
A Pitot-static tube is used to measure the velocity of helium in a pipe. The temperature and pressure are \(40^{\circ} \mathrm{F}\) and 25 psia. A water manometer connected to the Pitot-static tube...
-
Personality tests are useful tools for organizational hiring. Research suggests that applicants do fake to some degree on the tests, but faking does not significantly lower the correlation between...
-
Norma Smith is the controller of Baylor Corporation and is responsible for the preparation of the year-end financial statements. The following transactions occurred during the year. (a) On December...
-
Seven Question 17 Sponte The Bellevue firm will issue preferred stock with flotation costs of 5%. If the shares will sell for $48 and pay dividends of $1 the firm's cost of preferred as a percent to...
-
(A) Write Lewis structures for Br 2 , CH 4 , and HOCl. (B) Write Lewis structures for NI 3 , N 2 H 4 , and C 2 H 6 .
-
Explain why the first ionization energy of Mg is greater that of Na, whereas the second ionization of Na is greater than that of Mg.
-
The term corporate governance is frequently used, but few people truly understand what it means. How would you define it to aid in their understanding?
-
1. create a concept map for 0D, 1D, 2D and 3D crystals 2. write down the formulas for quantifying numbers of defects
-
\fNOTES TO CONSOLIDATED FINANCIAL STATEMENTS OF AMERICAN AIRLINES GROUP INC . Commitments , Contingencies and Guarantees ( 2 ) Aircraft and Engine Purchase Commitment Under all of our aircraft and...
-
Critical Values. In Exercises 41-44, find the indicated critical value. Round results to two decimal places. 41. Z0.25 42. Z0.90 43. Z0.02 44. 20.05
-
Use the following information for questions 1 and 2. Caterpillar Financial Services Corp. (a subsidiary of Caterpillar) and Sterling Construction sign a lease agreement dated January 1, 2020, that...
-
In todays social and business environments, some organizations only talk the talk regarding ethics and ethical conduct rather than walk the ethical organizational path. In what ways can ethical and...
-
Describe some hypothetical services that the network layer can provide to a single packet. Do the same for a flow of packets. Are any of your hypothetical services provided by the Internet's network...
-
In Exercises, find the equation of the tangent line at the given point on each curve. 2y 2 - x = 4; (16, 2)
-
What are the three major sections on a statement of cash flows, and what are the general rules that determine the transactions that should be included in each section?
-
They is interest paid on amounts borrowed from banks and other lenders considered to be an operating activity while the amounts borrowed are financing activities?
-
If an asset is sold at a gain, why is the gain deducted from net income when computing the net cash provided by operating activities under the indirect method?
-
S Corporation is expanding rapidly and it currently needs to retain all of its earnings. Hence, it does not pay any dividends. However, investors expect S Corp to begin paying dividends with the...
-
What is law accordingly to John Austin ? In what sense laws are different from morality? Discuss the importance of knowing commercial law for business executives.
-
Joey purchased a 14-year T-bond with a 3.5% annual coupon four years ago at par. Today the bond's YTM 5%. If Cramer holds this bond to maturity, what internal rate of return will he earn on this...

Study smarter with the SolutionInn App


