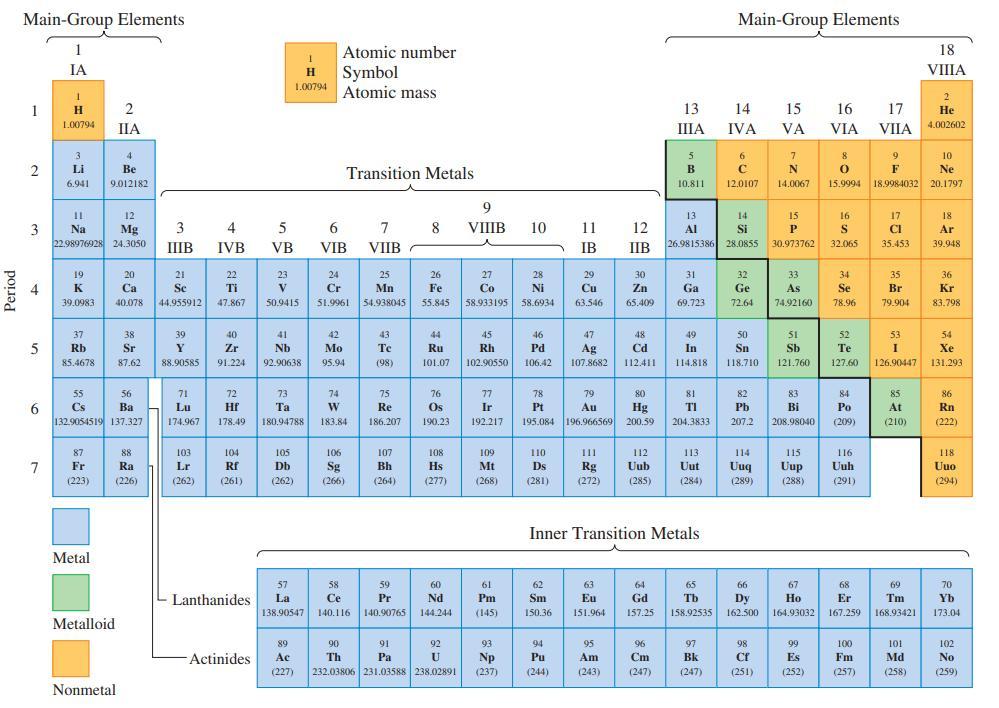
Refer only to the periodic table on the inside front cover and indicate which of the atoms,
Question:
Refer only to the periodic table on the inside front cover and indicate which of the atoms, Bi, S, Ba, As, and Ca,
(a) Is most metallic;
(b) Is most nonmetallic;
(c) Has the intermediate value when the five are arranged in order of increasing first ionization energy.
Transcribed Image Text:
Period Main-Group Elements 1 2 4 5 11 3 Na 6 IA 7 1 H 1.00794 3 Li 6.941 19 K 39.0983 12 Mg 22.98976928 24.3050 37 Rb 85.4678 55 Cs 87 Fr (223) 4 Be 9.012182 Metal 2 IIA 56 Ba 132.9054519 137.327 Metalloid 20 Ca 40.078 38 Sr 87.62 Nonmetal 88 Ra (226) 3 IIIB 21 Se 44.955912 39 Y 88.90585 71 Lu 174.967 103 Lr (262) 4 IVB 22 Ti 47.867 40 Zr 91.224 72 Hf 178.49 104 Rf (261) Lanthanides Actinides 5 VB 23 V 50.9415 1 H Symbol 1.00794 Atomic mass 41 Nb 92.90638 73 Ta 180,94788 105 Db (262) Atomic number 6 VIB Transition Metals 24 Cr 51.9961 42 Mo 95.94 74 W 183.84 106 Sg (266) 57 58 La Ce 138.90547 140.116 7 8 VIIB 25 Mn 54.938045 43 Te (98) 75 Re 186.207 107 Bh (264) 59 Pr 140.90765 26 Fe 55.845 91 Pa 44 Ru 101.07 76 Os 190.23 108 Hs (277) 60 Nd 144.244 92 89 Ac (227) 232.03806 231.03588 238.02891 90 Th U 9 VIIIB 27 Co 58.933195 45 Rh 102.90550 77 Ir 192.217 109 Mt (268) 61 Pm (145) 93 Np (237) 10 28 Ni 58.6934 46 Pd 106.42 110 Ds (281) 11 12 IB IIB 78 79 Pt Au 195.084 196.966569 62 Sm 150.36 29 Cu 63.546 94 Pu (244) 47 Ag 107.8682 30 Zn 65.409 63 Eu 151.964 48 Cd 112.411 111 112 Rg Uub (272) (285) 95 Am (243) 80 Hg 200.59 64 Gd 157.25 13 IIIA 96 Cm (247) 5 B 10.811 Inner Transition Metals 31 Ga 69.723 49 In 114.818 13 14 Al Si 26.9815386 28.0855 81 TI 204.3833 113 Uut (284) 65 Tb 158.92535 Main-Group Elements 97 Bk (247) 14 IVA 6 с 12.0107 32 Ge 72.64 50 Sn 118.710 82 Pb 207.2 114 Uuq (289) 66 Dy 162.500 98 Cf (251) 15 VA 7 N 14.0067 15 P 30.973762 33 As 74.92160 51 Sb 121.760 83 Bi 208.98040 115 Uup (288) 67 Ho 164.93032 99 Es (252) 16 VIA 8 9 0 F 15.9994 18.9984032 16 S 32.065 34 Se 78.96 52 Te 127.60 84 Po (209) 116 Uuh (291) 68 Er 167.259 17 VIIA 100 Fm (257) 17 CI 35.453 35 Br 79.904 53 I 126.90447 85 At (210) 69 Tm 168.93421 101 Md (258) 18 VIIIA 2 He 4.002602 10 Ne 20.1797 18 Ar 39.948 36 Kr 83.798 54 Xe 131.293 86 Rn (222) 118 Uuo (294) 70 Yb 173.04 102 No (259)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (3 reviews)
a Most Metallic Metals are typically found on the left side of the periodic table especially in the ...View the full answer

Answered By

PALASH JHANWAR
I am a Chartered Accountant with AIR 45 in CA - IPCC. I am a Merit Holder ( B.Com ). The following is my educational details.
PLEASE ACCESS MY RESUME FROM THE FOLLOWING LINK: https://drive.google.com/file/d/1hYR1uch-ff6MRC_cDB07K6VqY9kQ3SFL/view?usp=sharing
3.80+
3+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
Refer only to the periodic table on the inside front cover, and arrange the following ionization energies in order of increasing value: the first ionization energy of F; the second ionization energy...
-
(A) Refer only to the periodic table on the inside front cover, and arrange the following species in order of increasing size: Ti 2+ , V 3+ , Ca 2+ , Br - , and Sr 2+ . (B) Refer only to the periodic...
-
(A) Refer to the periodic table on the inside front cover, and arrange the following in the expected order of increasing first ionization energy Cl, K, Mg, S. (B) Refer to the periodic table on the...
-
31. Which combination of the compounds and their geometry are correct h) CIF, - V-shaped e) CIF, - Tshaped OB. a HeCl, - linoar 24. Which of the following havea bond angle smaller than tetrahodral...
-
How does the theory of comparative advantage relate to the currency swap market?
-
Air flows steadily along a streamline from point (1) to point (2) with negligible viscous effects. The following conditions are measured: At point (1) \(z_{1}=2 \mathrm{~m}\) and \(p_{1}=0...
-
Hofstedes taxonomy of cultural values includes individualism collectivism, power distance, uncertainty avoidance, masculinity femininity, short-term vs. long-term orientation, and indulgence vs....
-
Pierre Dupont just received a cash gift from his grandfather. He plans to invest in a five-year bond issued by Venice Corp. that pays an annual coupon of 5.5 percent. If the current market rate is...
-
What is non-cash expense (depreciation and other non cash expenses) and interest expense for 2019 ? Consolidated data Cons 31/12/2019 m USD 12 months Unqual US GAAP 10-K Income statement Total...
-
Refer to the periodic table on the inside front cover and indicate (a) The most nonmetallic element; (b) The transition metal with lowest atomic number; (c) A metalloid whose atomic number is exactly...
-
Explain why the first ionization energy of Mg is greater that of Na, whereas the second ionization of Na is greater than that of Mg.
-
If you are managing a DI that is technically insolvent but has not yet been closed by the regulators, would you invest in Treasury bonds or real estate development loans? Explain your answer.
-
reciprocal relationship between strategy and structure?
-
Steve Reese is a well - known interior designer in Fort Worth, Texas. He wants to start his own business and convinces Rob O Donnell , a local merchant, to contribute the capital to form a...
-
Use the following information for questions 1 and 2. Caterpillar Financial Services Corp. (a subsidiary of Caterpillar) and Sterling Construction sign a lease agreement dated January 1, 2020, that...
-
Porch Pirates An InsuranceQuotes.com survey showed that 8% of Americans had a holiday package stolen from outside their front door. Consider the random selection of four Americans. Use the...
-
GATE 2024-EE Question
-
What is the difference between a group-shared tree and a source-based tree in the context of multicast routing?
-
If (x) 0 on the interval [a, b], the definite integral gives the exact area under the curve between x = a and x = b.
-
Why arent transactions involving accounts payable considered to be financing activities?
-
Assume that a company repays a $300,000 loan from its bank and then later in the same year borrows $500,000. What amount(s) would appear on the statement of cash flows?
-
How do the direct and the indirect methods differ in their approach to computing the net cash provided by operating activities?
-
I need help trying to find this information for Kroger grocery stores. I need the most recent year, list the amounts reported for sales, cost of goods sold, and total net income. Does the amount...
-
Your grandmother gives you 2400 dollars for your birthday, which you invest in a mutual fund on January 1. On June 1, your fund balance is 7200 dollars, and you then deposit 1100 dollars (which you...
-
(Present-value comparison)You are offered $100,000 today or $360,000 in 13 years. Assuming that you can earn 12 percent on your money, which should you choose? If you are offered $360,000 in 13 years...

Study smarter with the SolutionInn App


