The crystal structure of lithium sulfide (Li 2 S), is pictured here. The length of the unit
Question:
The crystal structure of lithium sulfide (Li2S), is pictured here. The length of the unit cell is 5.88 x 102 pm. For this structure, determine
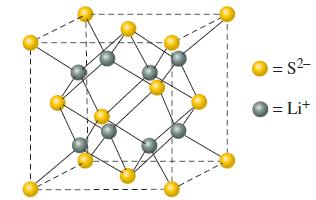
(a) The coordination numbers of Li+ and S2-;
(b) The number of formula units in the unit cell;
(c) The density of Li2S.
Transcribed Image Text:
= S²- = Lit
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (3 reviews)
a The coordination numbers of Li and S2 The coordination number of an ion is the number of nearest n...View the full answer

Answered By

Nazrin Ziad
I am a post graduate in Zoology with specialization in Entomology.I also have a Bachelor degree in Education.I posess more than 10 years of teaching as well as tutoring experience.I have done a project on histopathological analysis on alcohol treated liver of Albino Mice.
I can deal with every field under Biology from basic to advanced level.I can also guide you for your project works related to biological subjects other than tutoring.You can also seek my help for cracking competitive exams with biology as one of the subjects.
3.30+
2+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
The selection process is aimed at finding and hiring the best candidates for job openings. This process is often depicted as a funnel. For instance,50candidates may apply to a function, five of them...
-
A metal has a cubic structure with a density of 2.6 g/cm3, an atomic weight of 87.62 g/mol, and a lattice parameter of 6.0849 . One atom is associated with each lattice point. Determine the crystal...
-
A metal has a cubic structure with a density of 1.892 g/cm3, an atomic weight of 132.91 g/mol, and a lattice parameter of 6.13 . One atom is associated with each lattice point. Determine the crystal...
-
What are some examples of artificial selection? How are artificial selection and natural selection similar? How are they different?
-
McLaughlin borrowed $1,000 from Adler, who, apprehensive about McLaughlins ability to pay, demanded security. McLaughlin indorsed and delivered to Adler a negotiable promissory note executed by...
-
From the following transactions of E Stoddart in March 2022, complete the general journal using the perpetual inventory system. 1 Cash sale $110 ($100 + $10 GST), cost of sales $60. 3 Debit card...
-
The true test of authenticity! Can you have tough conversations and yet ensure that all save face?
-
Jabil Circuit, Inc., is a publicly traded electronics and technology company headquartered in St. Petersburg, Florida. In 2008, a group of shareholders who had owned Jabil stock from 2001 to 2007...
-
You are a credit analyst in the asset management department of a large bank or insurance company. The credit department is researching an investment in a syndicated loan to a large firm that used the...
-
The comparative balance sheets of American-Davis Design Studio, Inc., at June 30, 2018, and 2017, and transaction data for fiscal 2018, are as follows: Transaction data for the year ended June 30,...
-
Refer to Figure 12-44 and Figure 12-48. Suppose that the two planes of ions pictured in Figure 12-44 correspond to the top and middle planes of ions in the NaCl unit cell in Figure 12-48. If the...
-
The triple point temperature of bismuth is 544.5 K and the normal boiling point is 1832 K. Imagine that a 1.00 mol sample of bismuth is heated at a constant rate of 1.00 kJ min -1 in an apparatus in...
-
For two events, A and B, P(A) = .4, P(B) = .2, and P(A B) = .1: a. Find P(A | B). b. Find P(B | A). c. Are A and B independent events?
-
Perez Bags (PB) is a designer of high-quality backpacks and purses. Each design is made in small batches. Each spring, PB comes out with new designs for the backpack and for the purse. The company...
-
Find a recent (within the last 12 months) article or economic blog related to price fixing, provide an executive summary of the information. Include an APA reference and/or link. How does the fact...
-
A rectangular block of a material with a modulus of rigidity G=90 ksi is bonded to two rigid horizontal plates. The lower plate is fixed, while the upper plate is subjected to a horizontal force P....
-
A retail product has the following standard costs established: Direct Material per unit - 2 pounds at $5 a pound Direct Labor per unit - 3 hours at $12 an hour Manufacturing Overhead - $5 per labor...
-
In a recent year, the Better Business Bureau settled 75% of complaints they received. (Source: USA Today, March 2, 2009) You have been hired by the Bureau to investigate complaints this year...
-
As discussed in the "A Word About . . . NMR in Biology and Medicine" on page 368, MRI typically uses 1H NMR spectra of water in various tissues. What is the disadvantage of using 13C NMR spectra?...
-
How does Kant answer Humes bundle theory of self? Do you think he is successful?
-
Equity Multiplier and Return on Equity Bettles Fried Chicken Company has a debt-equity ratio of 0.80. Return on assets is 9.2 percent, and total equity is $520,000. What is the equity multiplier?...
-
Preparing Standardized Financial Statements prepare the 2006 and 2007 common-size balance sheets for Just Dew It. JUST DEW IT CORPORATION 2006 and 2007 Balance Sheets Liabilities and Owners' Equity...
-
Preparing Standardized Financial Statements prepare the 2007 commonbase year balance sheet for Just Dew It. JUST DEW IT CORPORATION 2006 and 2007 Balance Sheets Liabilities and Owners' Equity Assets...
-
Instructions Chart of Accounts UURILIUI LLUI ASSETS REVENUE The cash account for Coastal Bike Co. at October 1, 2099, indicated a balance of $34,800. During October, the total cash deposited was...
-
An employer has 6 employees, all of whom have exceeded the FUTA wage base. If the employer makes total payments to employees of $70,250 and enters $25,000 on line 5 of Form 940, then $__________ is...
-
Last month the average daily balance on Kaitlin's credit card was $1,180.81. If there were 31 days in that month, and her daily interest rate was 0.048%, what is the amount of interest that she will...

Study smarter with the SolutionInn App


