The emission spectrum below for a one-electron (hydrogen-like) species in the gas phase shows all the lines,
Question:
The emission spectrum below for a one-electron (hydrogen-like) species in the gas phase shows all the lines, before they merge together, resulting from transitions to the first excited state from higher energy states. Line A has a wavelength of 434 nm.
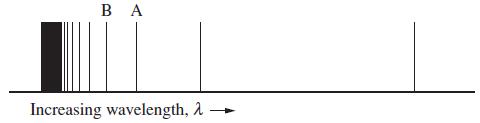
(a) What are the upper and lower principal quantum numbers corresponding to the lines labeled A and B?
(b) Identify the one-electron species that exhibits the spectrum.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:





