The following sketch is of a voltaic cell consisting of two standard electrodes for two metals, M
Question:
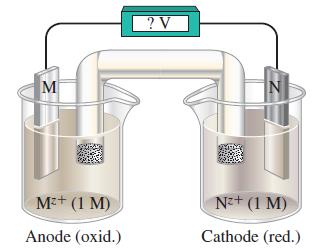
The following sketch is of a voltaic cell consisting of two standard electrodes for two metals, M and N:
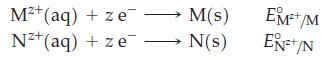
Use the standard reduction potentials of these half reactions to answer the questions that follow:
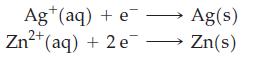
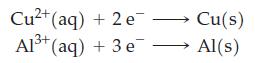
(a) Determine which pair of these half-cell reactions leads to a cell reaction with the largest positive cell potential, and calculate its value. Which couple is at the anode and which at the cathode?
(b) Determine which pair of these half-cell reactions leads to the cell with the smallest positive cell potential, and calculate its value. Which couple is at the anode and which is at the cathode?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:





