The following three-step mechanism has been proposed for the reaction of chlorine and chloroform. The numerical values
Question:
The following three-step mechanism has been proposed for the reaction of chlorine and chloroform.
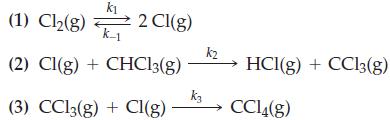
The numerical values of the rate constants for these steps are k1 = 4.8 x 103 ; k-1 = 3.6 x 103 ; k2 = 1.3 x 10-2; k3 = 2.7 x 102. Derive the rate law and the magnitude of k for the overall reaction.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:





