The overall reaction for the halogenation of a hydrocarbon (RH) using Br as the halogen is RH
Question:
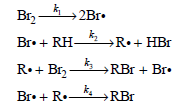
Determine the rate law predicted by this mechanism.
Transcribed Image Text:
Br2 2Br. Br• + RH- →R•+ HBr R•+ Brz -4 »RBr + Br• k. RBr Br• + R.-
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 64% (17 reviews)
Applying the steadystate approximation to the intermediate species Br and ...View the full answer

Answered By

Anjali Arora
Having the experience of 16 years in providing the best solutions with a proven track record of technical contribution and appreciated for leadership in enhancing team productivity, deliverable quality, and customer satisfaction. Expertise in providing the solution in Computer Science, Management, Accounting, English, Statistics, and Maths.
Also, do website designing and Programming.
Having 7 yrs of Project Management experience.
100% satisfactory answers.
5.00+
3+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Write the overall reaction for the fixation of nitrogen via the nitrogenase complex.
-
The overall reaction for the electrolytic production of aluminum by means of the Hall process may be represented as Al2O3 (s) + 3C (s) 2Al(l) + 3CO(g) At 1000C, the standard free-energy change for...
-
The overall reaction in the lead storage battery is Pb(s) + PbO2(s) + 2H+(aq) + 2HSO4-(aq) 2PbSO4(s) + 2H2O(l) d. Based on your previous answers, why does it seem that batteries fail more often on...
-
Below is the article related to the funding and construction of the SD Padres' ballpark in downtown San Diego. The article below relates to the Padres' decision to incur substantial payoff...
-
Restrict the domain of f (x) = x2 + 1 to x 0. Use a graphing utility to graph the function. Does the restricted function have an inverse function? Explain.
-
The figure below shows 2 tangent circles such that the 9- inch diameter of the smaller circle is equal to the radius of the larger circle. What is the approximate area, in square inches, of the...
-
Over the past three months, the demand for a product has been 255, 219, and 231. Calculate the three-month moving average forecast for month 4. If the actual demand in month 4 is 228, calculate the...
-
Danish Hospital recently installed a RAP Scanner, which is a diagnostic tool used both in suspected cancer cases and for detecting certain birth defects while the fetus is still in the womb. The...
-
Question 12 2020 Clank Ltd reported cost of goods sold as follows: 2019 Beginning inventory $ 64,000 $ 54,000 Cost of goods purchased 874,000 871.000 Cost of goods available for sale 938,000 925,000...
-
A company has decided to order 360 units whenever the on-hand inventory falls to 90 units. There appears to be no seasonal fluctuation to the demand, but it does fluctuate daily and is approximately...
-
The enzyme fumarase catalyzes the hydrolysis of fumarate: Fumarate (aq) + H 2 O(l) L-malate (aq) The turnover number for this enzyme is 2.5 10 3 s 1 , and the Michaelis constant is 4.2 10 6 M....
-
Consider the collision-induced dissociation of N2O5(g) via the following mechanism: The asterisk in the first reaction indicates that the reactant is activated through collision. Experimentally it is...
-
One advantage of using subscripted variables (an array) is that they take up fewer storage locations than the same number of unsubscripted variables. True or False
-
Q13. The probability that Ryan will roll a three using a standard die is 1/6. Let Y = number of times that Ryan has to roll a die in order to roll the first three. What is the expected value for Y?...
-
1. The following are data for two IT projects for a new database system. Prepare a spreadsheet for two projects, using the following data. Amounts are in thousands of dollars. Calculate the NPV for...
-
The Matsui Lubricants plant uses the weighted-average method to account for its work-in-process inventories. The accounting records show the following information for a particular day: Beginning WIP...
-
James Cook, a production department worker, is paid on hourly basis at a rate of $15 per hour. James works 40 hours per week. Any time James works over 40 hours, it is considered as overtime and he...
-
You just started working as a Health Service Manager within one of the following healthcare industries. First, choose an industry below to discuss the questions that follow: Ambulatory Surgery center...
-
Explain how to graph the reflection of y = f(x) across the x-axis. Give an example.
-
Draw a Feynman diagram for the reaction n + v p + .
-
Which of Ne or Ar has the larger van der Waals parameter a? Explain your reasoning.
-
From the following data at 298.15 K as well as data in Table 4.1 (Appendix B, Data Tables), calculate the standard enthalpy of formation of H 2 S(g) and of FeS 2 (s): AR(kJ mol) Fe(s) + 2H2S(g) ...
-
Which of Ne or Ar has the larger van der Waals parameter b? Explain your reasoning.
-
Imagine you are an Investor in the Stock Market. Identify three companies in the Korean Stock Market (KOSPI) where you would like to invest. Explain your answer
-
Domino is 4 0 years old and is married out of community of property with the exclusion of the accrual system to Dolly ( 3 5 ) . They have one child, Domonique, who is 1 1 years old. Domino resigned...
-
YOU ARE CREATING AN INVESTMENT POLICY STATEMENT FOR JANE DOE General: 60 years old, 3 grown children that are living on their own and supporting themselves. She is in a very low tax rate so we don't...

Study smarter with the SolutionInn App


