Consider the collision-induced dissociation of N2O5(g) via the following mechanism: The asterisk in the first reaction indicates
Question:
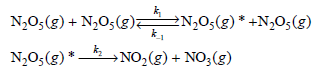
The asterisk in the first reaction indicates that the reactant is activated through collision.
Experimentally it is found that the reaction can be either first or second order in N2O5(g) depending on the concentration of this species. Derive a rate law expression for this reaction consistent with this observation.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





