Consider the following mechanism for ozone thermal decomposition: a. Derive the rate law expression for the loss
Question:
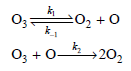
a. Derive the rate law expression for the loss of O3(g).
b. Under what conditions will the rate law expression for O3(g) decomposition be first order with respect to O3(g)?
Transcribed Image Text:
:02 +0 O3 03 +0- 202
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 85% (14 reviews)
a b Atomic oxygen is the intermediate species and the diffe...View the full answer

Answered By

Muhammad Mahtab
everyone looks that their work be perfect. I have more than a five year experience as a lecture in reputable institution, national and international. I provide perfect solution in marketing, case study, finance problems, blog writing, article writing, business plans, strategic management, human resource, operation management, power point presentation and lot of clients need. Here is right mentor who help clients in their multi-disciplinary needs.
5.00+
3+ Reviews
14+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Consider the following mechanism for renaturation of a double helix from its strands A and B: Derive the rate equation for the formation of the double helix and express the rate constant of the...
-
Consider the following mechanism for the thermal decomposition ofR2: Where R2, PA' PB are stable hydrocarbons and Rand R' are radicals. Find the dependence of the rate of decomposition of R, on the...
-
Consider the following mechanism for a reaction in aqueous solution and indicate the species acting as a catalyst: Explain why you believe this species is a catalyst. What is the overall reaction?...
-
Which of the following statements is correct regarding a work sheet and the adjustment process? Adjusting journal entries are prepared from the Adjusted Trial Balance columns. Adjusting journal...
-
Find the inverse function of f informally. Verify that f (f-1 (x)) = x and f-1(f (x)) = x. 1. f(x) = 6x 2. f(x) = 1/3x 3. f(x) = 3x + 1
-
A plane electromagnetic wave EI cos(k I z + I t) is incident on a perfectly reflecting mirror (solid line) that moves with constant velocity v = z. The reflected plane wave is ER cos(k R z R t)....
-
Using the data in problem 8.12 and the seasonal indices you have calculated, calculate expected monthly demand if the annual forecast is 2000 units. Month Seasonal Index Forecast January February...
-
1. Which of the qualities of successful entrepreneurs have Simeon and Turning Robe demonstrated? 2. Should Simeon and Turning Robe consider lowering their ingredient costs by switching to...
-
Question 1 of 4 - /5 View Policies Current Attempt in Progress Wayne Company is considering a long-term investment project called ZIP ZIP will require an investment of $128,838. It will have a useful...
-
A pulse from a laser of power 1mW lasts for 10ns. If the number of photons emitted per second is 3.491 x 10, calculate the wavelength of the laser.
-
Consider the formation of double-stranded (DS) DNA from two complementary single strands (S and S²) through the following mechanism involving an intermediate helix (IH): a. Derive the rate law...
-
Determine the expression for fractional coverage as a function of pressure for the dissociative adsorption mechanism described in the text in which adsorption is accompanied by dissociation: R,(g) +...
-
Identify and explain the four types of opinions an auditor may render.
-
IV. Normal Distribution 9. IQ scores are said to be normally distributed with a mean of 100 and a standard deviation of 15. Label the normal curve below and then answer the questions that follow. a....
-
Manually determine the range, variance, and standard deviation of the set of numbers. Show the computations: a. 3, 8, 10, 14, 9, 10, 12, 21, 5, 13, 11, 10 b. 232, 212, 151, 325, 142, 132, 142, 236,...
-
Alex and Bess have been in partnership for many years. The partners, who share profits and losses on a 70:30 basis, respectively, wish to retire and have agreed to liquidate the business. Liquidation...
-
1 Frequency Domain Analysis 1. Given an input u(t) = cos(t) + 2 sin(5t) cos(5t) which is a sum of a lower frequency signal and a higher-frequency noise. Determine the feasible range of time constant...
-
A motor supplies a constant torque or twist of M = 120 lb ft to the drum. If the drum has a weight of 30 lb and a radius of gyration of ko = 0.8 ft, determine the speed of the 15-lb crate A after it...
-
In Exercises 3140, write the standard form of the equation of the circle with the given center and radius. Center (2, -1), r = 4
-
1. What is the semi-annually compounded interest rate if $200 accumulates to $318.77 in eight years? Answer in percentage with two decimal places. 2. What is the quarterly compounded interest rate if...
-
Predict the products for each of the following reactions: a. b. c. d. . Ti[OCH(CH,),1. (+)-DET -- THCICH) (-)-DET
-
Benzoic acid, 1.35 g, is reacted with oxygen in a constant volume calorimeter to form H 2 O(l) and CO 2 (g) at 298 K. The mass of the water in the inner bath is 1.55 10 3 g. The temperature of the...
-
Calculate the P and T values for which Br2(g) is in a corresponding state to Xe(g) at 330. K and 72.0 bar.
-
Equipment with a book value of $84,000 and an original cost of $166,000 was sold at a loss of $36,000. Paid $100,000 cash for a new truck. Sold land costing $330,000 for $415,000 cash, yielding a...
-
The following is part of the computer output from a regression of monthly returns on Waterworks stock against the S&P 5 0 0 index. A hedge fund manager believes that Waterworks is underpriced, with...
-
Doisneau 25-year bonds have an annual coupon interest of 8 percent, make interest payments on a semiannual basis, and have a $1,000 par value. If the bonds are trading with a market's required yield...

Study smarter with the SolutionInn App


