Consider the formation of double-stranded (DS) DNA from two complementary single strands (S and S²) through the
Question:
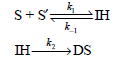
a. Derive the rate law expression for this reaction employing the preequilibrium approximation.
b. What is the corresponding rate law expression for the reaction employing the steady-state approximation for the intermediate IH?
Transcribed Image Text:
S+S=H →DS н-
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 44% (9 reviews)
a The rate law expression for the reaction is We apply the steadystate approximation to defin...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Consider the formation of atomic hydrogen in the reaction c + H+ = H, where e is an electron, as the adsorption of an electron on a proton H+. (a) Show that the equilibrium concentrations of the...
-
Consider the formation of nitrogen dioxide from nitric oxide and oxygen: 2NO(g) + O2(g) 2NO2(g) If 9.0 L of NO are reacted with excess O2 at STP, what is the volume in liters of the NO2 produced?
-
Consider the formation of glucose from carbon dioxide and water, that is, the reaction of the photosynthetic process: 6CO 2 (g) + 6H 2 O(l) C 6 H 12 O 6 (s) + 6O 2 (g). The following table of...
-
Pretty much any real object takes up space. This can cause confusion when asking, for instance, what is the r position of a car? Do we mean the front end of the car? The back end? Somewhere in...
-
Restrict the domain of the function f so that the function is one-to-one and has an inverse function. Then find the inverse function f-1. State the domains and ranges of f and f-1. Explain your...
-
Let () / 0 = 1 2 p / 2 be the dielectric function of the half-space z > 0. The half-space z (a) Relate to q in each medium. (b) Use D = 0 and the matching conditions for E to deduce that (c)...
-
Given the following average demand for each month, calculate the seasonal indices for each month. Month Average Demand Seasonal Index January 30 February 50 March 85 April 110 May 125 June 245 July...
-
State the effect (cash receipt or payment and amount) of each of the following transactions, considered individually, on cash flows: a. Sold equipment with a book value of $65,000 for $83,000. b....
-
3. (12 pts) Compute the balance in an account that earns 2.4% APR (annual nominal rate) if $5400 is deposited for 9 years with interest (round to the nearest cent): (a) compounded monthly (b)...
-
ZED plc manufactures one standard product, which sells at ?10. You are required to:(a) Prepare from the data given below, a break-even and profit?volume graph showing the results for the six months...
-
The hydrogenbromine reaction corresponds to the production of HBr(g) from H 2 (g) and Br 2 (g) as follows: H 2 (g) + Br 2 (g) 2HBr(g). This reaction is famous for its complex rate law, determined by...
-
Consider the following mechanism for ozone thermal decomposition: a. Derive the rate law expression for the loss of O 3 (g). b. Under what conditions will the rate law expression for O 3 (g)...
-
Describe the indirect method for determining net cash provided by operating activities.
-
Allison, Inc., produces two products, X and Y, in a single joint process. Last month the joint costs were P75,000 when 10,000 units of Product X and 15,000 units of Product Y were produced....
-
8+0.5 = 4. Consider a system with a lead compensator Ge(s) = +0.13 followed by a plant G(s) = 10 Determine a value for a gain K on the error signal such that the phase margin s(s+1) of the open-loop...
-
Standard Normal Distribution. In Exercises 17-36, assume that a randomly selected subject is given a bone density test. Those test scores are normally distributed with a mean of 0 and a standard...
-
After making several improvements in the east process, you now want to put controls in place to detect new problems if they occur. To help with this, the workers collected a sample of 5 capsules each...
-
Help es ! Required information The box shown has cardboard sides and wood strips along the edges and from corner to corner. The strength of the box is provided primarily by the wood strips, and a...
-
In Exercises 2738, graph each equation in a rectangular coordinate system. f(x) = x - 4
-
A 6-lb shell moving with a velocity ?? v0k explodes at point D into three fragments which hit the vertical wall at the points indicated. Fragments A, B, and C hit the wall 0.010 s, 0.018 s, and 0.012...
-
Assume that the equation of state for a gas can be written in the form P(V m b(T)) = RT. Derive an expression for = 1/V (V /T)P and = 1/V (V /P)T for such a gas in terms of b(T), db(T)/dT, P, and...
-
Consider the following two compounds. When treated with NaOH, one of these compounds forms an epoxide quite rapidly, while the other forms an epoxide very slowly. Identify which compound reacts more...
-
One mole of Ar initially at 310. K undergoes an adiabatic expansion against a pressure P external = 0 from a volume of 8.5 L to a volume of 82.0 L. Calculate the final temperature using the ideal gas...
-
4. The risk-free rate of return is 3.78% and the market risk premium is 6.42%. What is the expected rate of return on a stock with a beta of 1.09?
-
Maddox Resources has credit sales of $ 1 8 0 , 0 0 0 yearly with credit terms of net 3 0 days, which is also the average collection period. Maddox does not offer a discount for early payment, so its...
-
Selk Steel Co., which began operations on January 4, 2017, had the following subsequent transactions and events in its long-term investments. 2017 Jan. 5 Selk purchased 50,000 shares (25% of total)...

Study smarter with the SolutionInn App


