Question: Consider the following two compounds. When treated with NaOH, one of these compounds forms an epoxide quite rapidly, while the other forms an epoxide very
Consider the following two compounds. When treated with NaOH, one of these compounds forms an epoxide quite rapidly, while the other forms an epoxide very slowly. Identify which compound reacts more rapidly and explain the difference in rate between the two reactions.
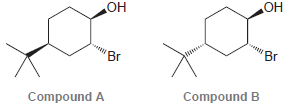
OH OH 'Br 'Br Compound B Compound A
Step by Step Solution
3.36 Rating (159 Votes )
There are 3 Steps involved in it
T his process for epoxide formation involves deprotonation of the hyd... View full answer

Get step-by-step solutions from verified subject matter experts


