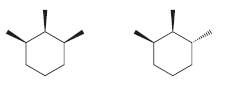
Consider the following two compounds. These compounds are stereoisomers of 1,2,3-trimethylcyclohexane. One of these compounds has three
Question:
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (7 reviews)
The first compound has three chirality centers This is apparent if we assi...View the full answer

Answered By

PALASH JHANWAR
I am a Chartered Accountant with AIR 45 in CA - IPCC. I am a Merit Holder ( B.Com ). The following is my educational details.
PLEASE ACCESS MY RESUME FROM THE FOLLOWING LINK: https://drive.google.com/file/d/1hYR1uch-ff6MRC_cDB07K6VqY9kQ3SFL/view?usp=sharing
3.80+
3+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Consider the following two compounds. When treated with NaOH, one of these compounds forms an epoxide quite rapidly, while the other forms an epoxide very slowly. Identify which compound reacts more...
-
Consider the following two compounds. How would you distinguish between them using: a) IR spectroscopy? b) 1 H NMR spectroscopy? c) 13 C NMR spectroscopy?
-
Consider the following two compounds: CH3CH2CH2CH2CH2OH 1-pentanol CH3CH2CH2CH2CH2CH3 hexane a. What are the different types of intermolecular forces that exist in each compound? b. One of these...
-
. What impacts of influences adult human behavior? Perspective Support: You must critically evaluate the issue and address the question: " What influences or impacts personality development?" by...
-
What role do finance companies play in leveraged buyouts?
-
Calculate the chi-square test statistic for the following datasets and null hypotheses a. H 0 : The population is distributed in a ratio of 1:2:1 for the categories A, B, and C, respectively. b. H 0...
-
Event A: Randomly select a female public school teacher. Event B: Randomly select a public school teacher who is 25 years old.
-
Selected accounts from the ledgers of Lockhart Company at July 31 showed the following. InstructionsFrom the data prepare:(a) The single-column purchases journal for July.(b) The general journal...
-
QUESTIONS ya The Company bases its predetermined overhead rate on the estimated direct labor hours for the year At the beginning of the most recently completed year, the Company estimated the direct...
-
If X and Y are independent random variables with means X = 9.5 and Y = 6.8 and standard deviations X = 0.4 and Y = 0.1, find the means and standard deviations of the following: a. 3X b. Y X c. X + 4Y
-
Cyclopropane is a compound in which the carbon atoms form a three-membered ring: Each of the carbon atoms in cyclopropane is sp 3 hybridized. Cyclopropane is more reactive than other cyclic compounds...
-
When butyl bromide is treated with sodium iodide in ethanol, the concentration of iodide quickly decreases but then slowly returns to its original concentration. Identify the major product of the...
-
The accompanying data represent x = amount of catalyst added to accelerate a chemical reaction and y 5 resulting reaction time: a. Calculate r. Does the value of r suggest a strong linear...
-
You want to retire after working 35 years with savings in excess of $1,100,000. You expect to save $3,300 a year for 35 years and earn an annual rate of Interest of 11%. (Round your answer to 2...
-
FOLLOW ALL INSTRUCTIONS AND GENERATE YOUR CODE AFTER READING THE JUNIT TESTS, THAT IS ALL THE METHODS AND CONSTRUCTORS YOU USE SHOULD BE BASED ON THE JUNIT TESTS PROVIDED. I HAVE ATTATCHED THE JAVA...
-
Are some values in the class data grossly different from all the others? If so, check for errors in calculation or procedure that would allow to objectively eliminate the data. 2. Do the range values...
-
An aging analysis of Uli Limited's accounts receivable at December 3 1 , 2 0 2 4 and 2 0 2 3 , showed the following: Number of Days Outstanding Accounts Receivable Estimated Percentage Uncollectible...
-
(Linear momentum) Two jets of liquid, one with specific gravity 1.00 and the other with specific gravity 1.33, collide and form one homogeneous jet as shown in the figure below. Determine (a) the...
-
Use f(x) and g(x) to find a formula for each expression. Identify its domain. (a) (f + g)(x) (c) (fg)(x) (b) (f- g)(x) (d) (f/g)(x)
-
From the choice of simple moving average, exponential smoothing, and linear regression analysis, which forecasting technique would you consider the most accurate? Why? please write it in word...
-
What products would you expect from oxidation of the following compounds with CrO3 in aqueous acid with pyridinium chlorochromate? (a) 1-Hexanol (b) 2-Hexanol (c) Hexanol
-
TMS ethers can be removed by treatment with fluoride ion as well as be acid-catalyzed hydrolysis. Propose a mechanism for the reaction of cyclohexyl TMS ether with LiF. Fluorotrimethyisilane is a...
-
Show the mechanism of the reaction of p-methaylphenol with 2-methylpropene and H3PO4 catalyst to yield the food additive BHT.
-
Arnold inc. is considering a proposal to manufacture high end protein bars used as food supplements by body builders. The project requires an upfront investment into equipment of $1.4 million. This...
-
Billy Bob bank has three assets. It has $83 million invested in consumer loans with a 3-year duration, $46 million invested in T-Bonds with a 12-year duration, and $69 million in 6-month (0.5 years)...
-
Ventaz Corp manufactures small windows for back yard sheds. Historically, its demand has ranged from 30 to 50 windows per day with an average of 4646. Alex is one of the production workers and he...

Study smarter with the SolutionInn App


