Cyclopropane is a compound in which the carbon atoms form a three-membered ring: Each of the carbon
Question:
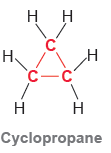
Each of the carbon atoms in cyclopropane is sp3 hybridized. Cyclopropane is more reactive than other cyclic compounds (four-membered rings, five-membered rings, etc.). Analyze the bond angles in cyclopropane and explain why cyclopropane is so reactive.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





