What alcohols would give the following products on oxidation? (b) (c) C (a) C
Question:
What alcohols would give the following products on oxidation?
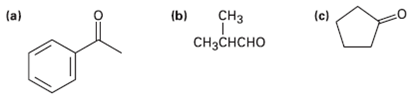
Transcribed Image Text:
(b) (c) Cнз (a) Cнзснсно
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 57% (19 reviews)
Strategy Aldehydes are synthesized from oxid...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
What starting material would give the following compound in an aliphatic Claisen rearrangement? CH,-CH-C
-
Give structures of the alkenes that would give the following products upon ozonolysis-reduction. (a) (b) and CH,CH,CH,_C_H cyclohexanone CH,-CH-_-_CH
-
The enzymatic oxidation of alkanes to produce alcohols is a simplified version of the reactions that produce the adrenocortical steroid hormones. In the biosynthesis of corticosterone from...
-
On 13 May 2014, Ruben acquired 400 shares in Xantan Ltd at a cost of 1,800. On 17 July 2020, the company made a 1 for 8 bonus issue and (on the same day) Ruben sold his bonus shares for 7 each....
-
What does "patching" refer to? Describe and illustrate two rules that might guide managers to build value in their businesses.
-
Entrepreneurs are important because of the impact they have on the world around them. List and explain the different ways that Fifteen benefits its community.
-
2. Does Pop use a one-line consolidation in accounting for its investment in Son? Explain your answer.
-
Given the aggression scores below for Outcome A of the sleep deprivation experiment, verify that, as suggested earlier, these mean differences shouldnt be taken seriously by testing the null...
-
Purchases for a company for January, February, and March are forecasted to be as follows: January: $ 2 5 0 , 0 0 0 February: $ 4 5 0 , 0 0 0 March: $ 5 5 0 , 0 0 0 respectively. Thirty percent of...
-
A store maintains data on customers, products and purchase records in three tables: CUSTOMER, PRODUCT, PURCHASE. The store manager wants to know which product is on its maximum discount for each...
-
What product(s) would you expect from dehydration of the following alcohols with POCl3 in pyridine? Indicate the major product in eachcase? (a) (b) (c) CH3CH2CHCHCH3 CH CH3 H (d) (e) CCCH2CH...
-
What products would you expect from oxidation of the following compounds with CrO3 in aqueous acid with pyridinium chlorochromate? (a) 1-Hexanol (b) 2-Hexanol (c) Hexanol
-
Consider the supply of shipping services. The law of supply suggests that as the price of shipping increases, the quantity of shipping services will increase. At a relatively low freight rate of $2...
-
Problem 1 PROBLEMS Sabres Limited, a Canadian-controlled private corporation whose fiscal year end is December 31, provides you with the following data concerning its tax accounts and capital...
-
9.6. A habitual gambler often visits three different casinos and plays roulette there. He wants to discover at which casino he has better luck with his roulette games. So, he records his gambling...
-
The firm has estimated that its sales for 2 0 1 3 will be $ 8 4 6 , 7 5 6 Cash dividends to be paid by the firm in 2 0 1 3 $ 3 7 , 7 2 0 Minimum cash balance to be maintained by the firm $ 2 8 , 5 1...
-
Bob Long was hired by County Hospital aS supervisor of engineering and maintenance. Although well experienced in his field, this was his first management job. Soon after Bob's arrival a maintenance...
-
Initial Outlay (IO) 1. A company is considering purchasing a machine for $100,000. Shipping costs would be another $5,000. The project would require an initial investment in net working capital of...
-
Watson Walbert has been the Executive Chef at the Altina Restaurant for five years. Altina Restaurant is located in a dry area (alcoholic beverage sales are illegal), so it only sells food. In the...
-
Avatar Financials, Inc., located on Madison Avenue, New York City, is a company that provides financial advice to individuals and small- to mid-sized businesses. Its primary operations are in wealth...
-
Of the following liquids at 20 C, which has the smallest viscosity? (a) Dodecane, C 12 H 26 ; (b) n-nonane, C 9 H 20 ; (c) n-heptane, C 7 H 16 ; (d) n-pentane, C 5 H 12 .
-
Using the pKa values of analogous compounds in Table 3.1, predict which would be the stronger base. (a) (b) (c) (d) or (CHa),Cor O: HO or
-
The pKa of the anilinium ion (C6H5NH3) is 4.63. On the basis of this fact, decide whether aniline (C6H5NH2) is a stronger or weaker base than methylamine.
-
Predict the outcome of the following reaction. -NH2
-
why should Undertake research to review reasons for previous profit or loss?
-
A pension fund's liabilities has a PV01 of $200 million. The plan has $100 billion of assets with a weighted average modified duration of 8. The highest duration bond that the plan can invest in has...
-
Metlock Limited has signed a lease agreement with Lantus Corp. to lease equipment with an expected lifespan of eight years, no estimated salvage value, and a cost to Lantus, the lessor of $170,000....

Study smarter with the SolutionInn App


