Show that the following mechanism is consistent with the rate law established for the iodidehypochlorite reaction in
Question:
Show that the following mechanism is consistent with the rate law established for the iodide–hypochlorite reaction in Exercise 79.
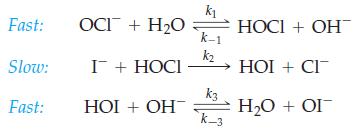
Exercise 79
Hydroxide ion is involved in the mechanism of the following reaction but is not consumed in the overall reaction.
![]()
(a) From the data given, determine the order of the reaction with respect to OCl-, I-, and OH-.
(b) What is the overall reaction order?
(c) Write the rate equation, and determine the value of the rate constant, k.
![[OCI ], M 0.0040 0.0020 0.0020 0.0020 0.0020 Rate Formation [I], M 0.0020 1.00 0.0040 1.00 0.0020 1.00 0.0020](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1701/0/6/0/16465641e44d0eff1701060162999.jpg)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:





