The following very strong acids are formed by the reactions indicated: (a) Identify the Lewis acids and
Question:
The following very strong acids are formed by the reactions indicated:
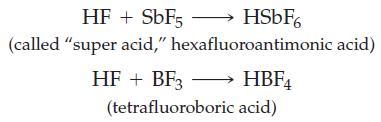
(a) Identify the Lewis acids and bases.
(b) To which atom is the H atom bonded in each acid?
Transcribed Image Text:
HF + SbF5- (called "super acid," HSbF6 hexafluoroantimonic acid) HF + BF3 →→→→ HBF4 (tetrafluoroboric acid)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 85% (7 reviews)
a Identify the Lewis acids and bases HF SbF5 HSbF6 In this reactionHF is the Lew...View the full answer

Answered By

PALASH JHANWAR
I am a Chartered Accountant with AIR 45 in CA - IPCC. I am a Merit Holder ( B.Com ). The following is my educational details.
PLEASE ACCESS MY RESUME FROM THE FOLLOWING LINK: https://drive.google.com/file/d/1hYR1uch-ff6MRC_cDB07K6VqY9kQ3SFL/view?usp=sharing
3.80+
3+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
Use the concept of hybrid orbitals to describe the bonding in the strong acids given in Exercise 78. Exercise 78 The following very strong acids are formed by the reactions indicated: (a) Identify...
-
An investor wishes to analyse the effects of different compounding frequencies Suppose 1000 is invested for 1 year at an interest rate of 5 per annum compounded Assume there are 365 days in 1 year
-
In the following acid-base reactions, 1. Determine which species are acting as electrophiles (acids) and which are acting as nucleophiles (bases). 2. Use the curved-arrow formalism to show the...
-
For what number does the principal square root exceed eight times the number by the largest amount?
-
Briefly describe each of the following takeover defenses against a hostile merger: (a) White knight, (b) Poison pill, (c) Greenmail, (d) Leveraged recapitalization, (e) Golden parachutes, and (f)...
-
A listing of 46 mutual funds and their 12-month total return percentage is shown in Table 3.5 (Smart Money, February 2004). a. What are the mean and median return percentages for these mutual funds?...
-
What is a perpetual project? Why might an organization be reluctant to terminate a project that many would consider unsuccessful? AppendixLO1
-
Foghorn Leghorn is considering the replacement of an old egg-sorting machine used with his Foggy's Farm Fresh Eggs business. The old ,egg machine is not quite running eggs actly the way it was...
-
the set of assumptions underlying the firm's financial plan and the resulting projected financial statements are accordingly often referred to as which of the following? 1. Base case projections. 2....
-
The molecular solid I 2 (s) is only slightly soluble in water but will dissolve to a much greater extent in an aqueous solution of KI, because the I 3 - anion forms. Write an equation for the...
-
CO 2 (g) can be removed from confined quarters (such as a spacecraft) by allowing it to react with an alkali metal hydroxide. Show that this is a Lewis acidbase reaction. For example, CO(g) + LiOH(s)...
-
The demand for snorkels in Berhama is given by Q S = 500 8P S and the supply of snorkels in Berhama is given by Q S = 200 + 4P S . The demand for kayaks is given by Q k = 650 6P k and the supply of...
-
Question: 9. Purchases and sales during a recent period for Bottineau Inc. were Purchases During the Period Sales During the Period 1st purchase 1,500 units x $ 4 1st sale 700 units x $13 2nd...
-
# The following is a partial relative frequency distribution of consumer preferences for four products-A, B, C, and D. Required: Determine the relative frequency for Product B: Relative Frequency...
-
Domino Company's operating percentages were as follows: Revenues 100% Cost of goods sold Variable 50% Fixed 10% 60% Gross profit 40% Other operating expenses Variable 20% Fixed 15% 35% Operating...
-
Marcus Stewart, the production manager at Galvin Company, purchased a cutting machine for the company last year. Six months after the purchase of the cutting machine, Stewart learned about a new...
-
The TechTeach Company produces and sells 7,000 modular computer desks per year at a selling price of $750 each. Its current production equipment, purchased for $1,950,000 and with a 5-year useful...
-
The president of Murquery Company is puzzled. During the last year, the company experienced a net loss of $800,000, yet its cash increased $300,000 during the same period of time. Explain to the...
-
On July 1, 2011, Flashlight Corporation sold equipment it had recently purchased to an unaffiliated company for $480,000. The equipment had a book value on Flashlights books of $390,000 and a...
-
Determine the income participation of Hassell and Lawson, according to each of the five assumptions as to income division listed in Exercise 12-3 if the years net income is $380,000.
-
Casey Fisher and Logan Baylor formed a partnership in which the partnership agreement provided for salary allowances of $40,000 and $35,000, respectively. Determine the division of a $20,000 net loss...
-
Ben Bowman and Savannah Mapes formed a limited liability company with an operating agreement that provided a salary allowance of $75,000 and $60,000 to each member, respectively. In addition, the...
-
When preparing government-wide financial statements, the modified accrual based governments funds are adjusted. Please show the adjustments (in journal entry form with debits and credits) that would...
-
I need help finding the callable price and call value
-
On 31 October 2022, the owner took goods for his son as a birthday gift. The cost price of the goods was R15 000

Study smarter with the SolutionInn App


