The laboratory device pictured on the following page is called a desiccator. It can be used to
Question:
The laboratory device pictured on the following page is called a desiccator. It can be used to maintain a constant relative humidity within an enclosure. The material(s) used to control the relative humidity are placed in the bottom compartment, and the substance being subjected to a controlled relative humidity is placed on the platform in the container.

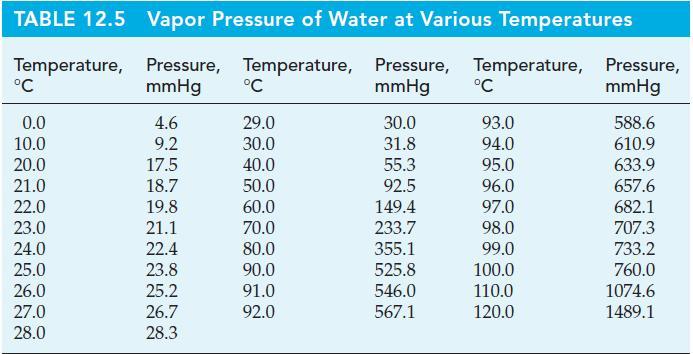
(a) If the material in the bottom compartment is a saturated solution of NaCl(aq) in contact with NaCl(s), what will be the approximate relative humidity in the container at a temperature of 20 °C? Obtain the solubility of NaCl from Figure 14-10 and vapor pressure data for water from Table 12.5; use the definition of Raoult’s law and that of relative humidity from Focus: Earth’s Atmosphere; assume that the NaCl is completely dissociated into its ions.
(b) If the material placed on the platform is dry CaCl2 · 6 H2O(s), will the solid deliquesce? Explain.
(c) To maintain CaCl2 · 6 H2O(s) in the dry state in a desiccator, should the substance in the saturated solution in the bottom compartment be one with a high or a low water solubility? Explain.
Figure 14-10
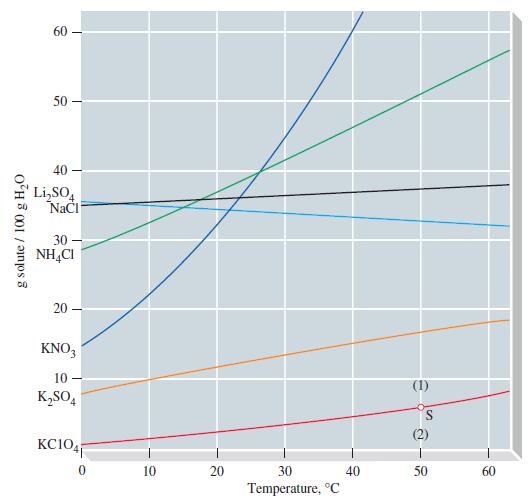
Table 12.5
Step by Step Answer:

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette





