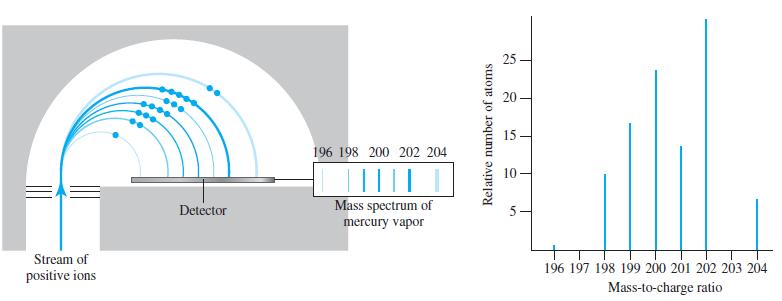
The masses of the naturally occurring mercury isotopes are 196 Hg, 195.9658 u; 198 Hg, 197.9668 u;
Question:
The masses of the naturally occurring mercury isotopes are 196Hg, 195.9658 u; 198Hg, 197.9668 u; 199Hg, 198.9683 u; 200Hg, 199.9683 u; 201Hg, 200.9703 u; 202Hg, 201.9706 u; and 204Hg, 203.9735 u. Use these data, together with data from Figure 2-14, to calculate the weighted-average atomic mass of mercury.
Figure 2-14
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:





