The packing fraction of a nuclide is related to the fraction of the total mass of a
Question:
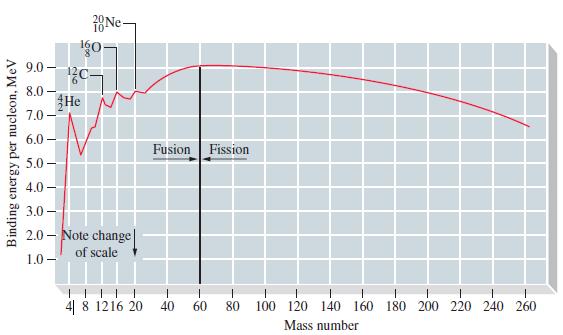
The packing fraction of a nuclide is related to the fraction of the total mass of a nuclide that is converted to nuclear binding energy. It is defined as the fraction 1M - A2/A, where M is the actual nuclidic mass and A is the mass number. Use data from a handbook (such as the Handbook of Chemistry and Physics, published by the CRC Press) to determine the packing fractions of some representative nuclides. Plot a graph of packing fraction versus mass number, and compare it with Figure 25-6. Explain the relationship between the two.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:





