The solubility of Cl 2 (g) in water is 6.4 g L 1 at 25 C. Some
Question:
The solubility of Cl2(g) in water is 6.4 g L–1 at 25 °C. Some of this chlorine is present as Cl2, and some is found as HOCl or Cl–. For the hydrolysis reaction
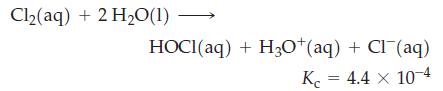
For a saturated solution of Cl2 in water, calculate [Cl2], [HOCl], [H3O+], and [Cl–].
Transcribed Image Text:
Cl₂(aq) + 2 H₂O(1) HOCI(aq) + H3O+(aq) + Cl(aq) Kc = 4.4 x 10-4
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 85% (7 reviews)
To calculate the concentrations of the various species in a saturated solution of Cl2 in water we ca...View the full answer

Answered By

Dinesh F
I have over 3 years of professional experience as an assignment tutor, and 1 year as a tutor trainee.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
The solubility of Cl2 in 100 g of water at STP is 310 cm3. Assume that this quantity of Cl2 is dissolved and equilibrated as follows: (a) If the equilibrium constant for this reaction is 4.7 Ã...
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
A sample containing an alkali sulfate is dried, weighed and dissolved in dilute HCl. Barium chloride solution is added in excess to precipitate barium sulfate, and the precipitate is digested in the...
-
On April 1, 2014, Briggs Corp. purchases a 24-month property insurance policy for $72,000. The policy is effective immediately. Assume that Briggs prepares adjusting entries only once a year, on...
-
You are an analyst for an investment fund that invests in initial public offerings (IPOs). You are looking at the financial statements of two companies, Clark Company and Durfee Company, that plan to...
-
Laelle Airlines, an international airlines company, offers a variety of services to its customers. Given this information, which of the following is the core service provided by Laelle Airlines? 1....
-
Rectify the following errors: (a) Salary paid to Mohan has been debited to his personal account ` 300. (b) ` 1500 withdrawn by the proprietor has been debited to the general expenses account. (c)...
-
Three peopleBaker, Adams, and Nelsonorganized Dog-Eared Book Shoppe, Inc. The charter of this corporation authorizes capital consisting of the following: a. 2,600 shares of preferred 9 percent stock,...
-
Assume that the City of Coyote has produced its financial statements for December 31, 2020, and the year then ended. The city's general fund was only used to monitor education and parks. Its capital...
-
Not shown in Figure 22-17 are electrode potential data involving hydrazoic acid. Given that E = -3.09 V for the reduction of HN 3 to N 2 in acidic solution, what is E for the reduction of HN 3 to NH...
-
Write plausible half-equations and a balanced oxidationreduction equation for the disproportionation of XeF 4 to Xe and XeO 3 in aqueous acidic solution. Xe and XeO 3 are produced in a mole ratio,...
-
A study of selected Kickstarter projects showed that overall a majority were successful, achieving their goal and raising, at a minimum, the targeted amounts. In an effort to identify project types...
-
Problem 1 PROBLEMS Sabres Limited, a Canadian-controlled private corporation whose fiscal year end is December 31, provides you with the following data concerning its tax accounts and capital...
-
9.6. A habitual gambler often visits three different casinos and plays roulette there. He wants to discover at which casino he has better luck with his roulette games. So, he records his gambling...
-
The firm has estimated that its sales for 2 0 1 3 will be $ 8 4 6 , 7 5 6 Cash dividends to be paid by the firm in 2 0 1 3 $ 3 7 , 7 2 0 Minimum cash balance to be maintained by the firm $ 2 8 , 5 1...
-
Bob Long was hired by County Hospital aS supervisor of engineering and maintenance. Although well experienced in his field, this was his first management job. Soon after Bob's arrival a maintenance...
-
Initial Outlay (IO) 1. A company is considering purchasing a machine for $100,000. Shipping costs would be another $5,000. The project would require an initial investment in net working capital of...
-
If B is a Boolean algebra, partially ordered by , and x, y B, what is the dual of the statement "x y"?
-
The vapor pressure of the liquid NH, is measured at different temperatures. The following vapor pressure data are obtained. Temperature, K P, mmHg 217.1 223.4 234.7 588.1 Calculate the enthalpy of...
-
The following information was taken from the 2009 financial statements of FedEx Corporation, a major global transportation/delivery company. InstructionsAnswer each of the following questions.(a)...
-
The following ratios are available for Tym Inc. Instructions(a) Is Tym??s short-term liquidity improving or deteriorating in 2012? Be specific in your answer, referring to relevant ratios.(b) Do...
-
On March 3, Pitrof Appliances sells $710,000 of its receivables to American Factors Inc. American Factors Inc. assesses a service charge of 4% of the amount of receivables sold. Instructions Prepare...
-
Physical Units Method, Relative Sales Value Method Farleigh Petroleum, Inc., is a small company that acquires high - grade crude oil from low - volume production wells owned by individuals and small...
-
A proposed $2.5 M investment in new equipment at a 100 MG/y M&Ms factory will save the plant $800,000/y in energy costs. Assuming an annual interest rate of 5%/y (compounded annually), and an...
-
Brief Exercise 10-7 Coronado Company obtained land by issuing 2,250 shares of its $14 par value common stock. The land was recently appraised at $103,240. The common stock is actively traded at $44...

Study smarter with the SolutionInn App


