The standard enthalpy of fermentation of glucose to ethanol is Use the standard enthalpy of combustion for
Question:
The standard enthalpy of fermentation of glucose to ethanol is
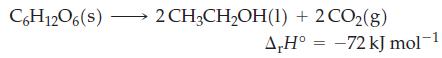
Use the standard enthalpy of combustion for glucose to calculate the enthalpy of combustion for ethanol.
Transcribed Image Text:
C6H12O6(s)- 2 CH3CH₂OH(1) + 2 CO₂(g) A,H° -72 kJ mol-1
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 85% (7 reviews)
The enthalpy change for a chemical reaction is given by the difference in the standard enthal...View the full answer

Answered By

OTIENO OBADO
I have a vast experience in teaching, mentoring and tutoring. I handle student concerns diligently and my academic background is undeniably aesthetic
4.30+
3+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
Ethanol (C2H5OH) has been proposed as an alternative fuel. Calculate the standard enthalpy of combustion per gram of liquid ethanol.
-
The standard enthalpy of combustion of solid urea (CO (NH2)2) is -632 kl mol-1 at 298 K and its standard molar entropy is 104.60 J K-1 mol-1, Calculate the standard Gibbs energy of formation of urea...
-
The standard enthalpy of combustion of ethene gas [C2H4(g)] is 1411.1 kJ/ mol at 298 K. Given the following enthalpies of formation, calculate Hof for C2H4(g). CO2(g) 393.5 kJ/ mol H2O(l) 285.8 kJ/...
-
The following e-mail message contains numerous errors related to what you've learned about planning and writing business messages. Using the information it contains, write a more effective version....
-
Shown here are annual financial data at December 31, 2009, taken from two different companies. Required 1. Compute the cost of goods sold section of the income statement at December 31, 2009, for...
-
A year ago, Rebecca purchased 100 shares of Havad stock for $25 per share. Yesterday, she placed a limit order to sell her stock at a price of $30 per share before the market opened. The stocks price...
-
CAPITAL BUDGETING CRITERIA: ETHICAL CONSIDERATIONS An electric utility is considering a new power plant in northern Arizona. Power from the plant would be sold in the Phoenix area, where it is badly...
-
Pickeril Inc. issues a $600,000, 10%, 10-year mortgage note on December 31, 2010, to obtain financing for a new building. The terms provide for semiannual installment payments of $48,145. Prepare the...
-
Imagine that you are a financial manager researching investments for your client. Think of a friend or a family member as a client. Define their characteristics and goals such as an employee or...
-
A company conducts a consumer survey with questions about home ownership (OwnHome: Yes/No), car ownership (OwnCar: Yes/No), annual household spending on food (Food), and annual household spending on...
-
Use the information given here, data from Appendix D, and equation (7.22) to calculate the standard enthalpy of formation per mole of ZnS(s). Eq.7.22 2 ZnS(s) + 3O(g) 2 ZnO(s) + 2 SO(g) A,H -878.2 kJ...
-
Use standard enthalpies of formation from Tables 7.2 and 7.3 and equation (7.22) to determine the standard enthalpy of reaction in the following reaction. Tables 7.2 Tables 7.3 Eq. 7.22 NH4+ (aq) +...
-
In Problems 942, find each limit algebraically. x4 2 lim x-2x - 2x
-
2. DETAILS MY NOTES In a statistical test, we have a choice of a left-tailed test, a right-tailed test, or a two-tailed test. Is it the null hypothesis or the alternate hypothesis that determines...
-
2. The model of a two-story building shown in Figure 2. The girders are assumed to be rigid, and the columns have flexural rigidities EI and EI2, with negligible masses. The stiffness of each column...
-
Prepare journal entries to record these transactions. (List all debit entries before credit entries. Credit account titles are automatically indented when amount is entered. Do not indent manually....
-
Popcorn company is expected to pay $1 dividend per share at the end of this year, $1.50 dividend per share at the end of year 2, $2 dividend per share at the end of year 3, and $2.50 dividend per...
-
Increased spending for COVID economic relief is an important issue for many struggling in the current economy. A specific policy to combat this issue is put forward and it is found that 78% of...
-
What are the chief functions of cutting fluids?
-
In the synthesis of the keto acid just given, the dicarboxylic acid decarboxylates in a specific way; it gives Explain. HO rather than HO
-
What is the indirect effect of a change in accounting principle? Briefly describe the reporting of the indirect effects of a change in accounting principle.
-
Define a change in estimate and provide an illustration. When is a change in accounting estimate effected by a change in accounting principle?
-
Lenexa State Bank has followed the practice of capitalizing certain marketing costs and amortizing these costs over their expected life. In the current year, the bank determined that the future...
-
What do you consider as, potentially, the most important elements when working to improve an organizations competitive advantage and create value for all stakeholders?
-
Ch . 5 - Quick Study 4 A company reports the following beginning inventory and two purchases for the month of January. On January 2 6 , the company sells 3 6 0 units. Ending inventory at January 3 1...
-
A deferred tax liability is created when Select one: a. the book basis of assets is greater than the tax basis of assets. b. the book basis of liabilities is greater than the tax basis of...

Study smarter with the SolutionInn App


