Use standard enthalpies of formation from Tables 7.2 and 7.3 and equation (7.22) to determine the standard
Question:
Use standard enthalpies of formation from Tables 7.2 and 7.3 and equation (7.22) to determine the standard enthalpy of reaction in the following reaction.
![]()
Tables 7.2
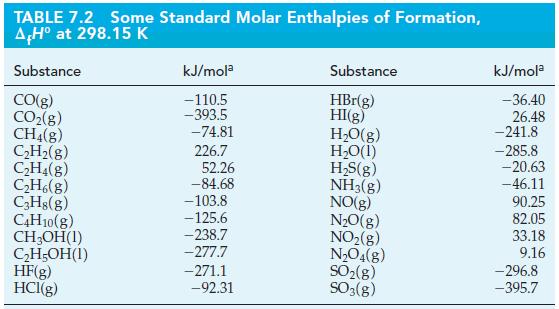
Tables 7.3
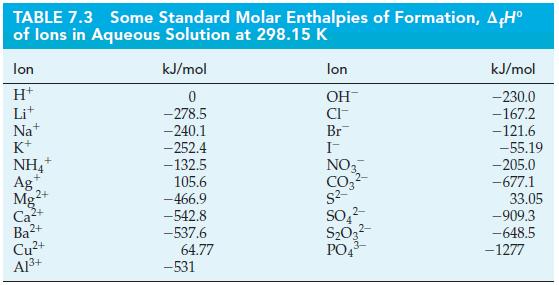
Eq. 7.22![A,H [cx AHc + dx AHD + = weighted sum of A H values for the products .] [ax AHA + bx AfH B + weighted sum of](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1699/6/0/4/555654de84b5bbd41699604550819.jpg)
Transcribed Image Text:
NH4+ (aq) + OH(aq) H₂O(1) + NH3(g).
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 80% (5 reviews)

Answered By

Muqadas Javed
I am a mentor by profession since seven years. I have been teaching on online forums and in universities. Teaching is my passion therefore i always try to find simple solution for complicated problems or task grasp them so that students can easily grasp them.I will provide you very detailed and self explanatory answers and that will help you to get good grade. I have two slogans: quality solution and on time delivery.
4.60+
24+ Reviews
144+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
Use standard enthalpies of formation from Table 7.2 and equation (7.22) to determine the standard enthalpy of reaction in the following reactions. Table 7.2 Eq. 7.22 (a) C3H8(g) + H(g) CH6(g) +...
-
The standard enthalpies of formation of ClO and ClO2 are 101 and 102 kJ/mol, respectively. Using these data and the thermodynamic data in Appendix C, calculate the overall enthalpy change for each...
-
(a) Why are tables of standard enthalpies of formation so useful? (b) What is the value of the standard enthalpy of formation of an element in its most stable form? (c) Write the chemical equation...
-
Tom Jones, the mechanic at Golden Muffler Shop, is able to install new mufflers at an average rate of 4 per hour (or about 1 every 15 minute), according to a negative exponential distribution....
-
Laredo Boot Company makes specialty boots for the rodeo circuit. On December 31, 2008, the company had (a) 300 pairs of boots in finished goods inventory and (b) 1,400 heels at a cost of $16 each in...
-
What is an open end mutual fund? What types of companies usually manage open end funds? Describe how these funds work on a day today basis.
-
CAPITAL BUDGETING CRITERIA: ETHICAL CONSIDERATIONS A mining company is considering a new project. Because the mine has received a permit, the project would be legal; but it would cause significant...
-
Using the Public MACRO BITCOIN scorecard spreadsheet (linked in its associated masterclass lesson - Long Term 32), create a COPY of it and perform a complete analysis for the date 22/2/2022....
-
A professor has two daughters that he hopes will one day go to college. Currently, in-state students at the local University pay about $22,044.00 per year (all expenses included). Tuition will...
-
Given the following project network and baseline information below, complete the form to develop a status report for the project at the end of period 4 and the end of period 8. 0 A 2 0 2 2 2 O Task A...
-
The standard enthalpy of fermentation of glucose to ethanol is Use the standard enthalpy of combustion for glucose to calculate the enthalpy of combustion for ethanol. C6H12O6(s)- 2 CH3CHOH(1) + 2...
-
One glucose molecule, C 6 H 12 O 6 (s), is converted to two lactic acid molecules, CH 3 CH(OH)COOH(s) during glycolysis. Given the combustion reactions of glucose and lactic acid, determine the...
-
You want a retirement fund of $125,000 when you retire in six years. You are able to earn 8 percent on your investments. How much should you deposit each year to build the retirement fund that you...
-
James worked a total of 186 hours for the month of June 2020. His rate per hour is working hours of the 450 per hour. Overtime premium is 30%. The company is 8 hours a day. The company's regular...
-
Question Researchers collected a simple random sample of 36 children who had been identified as gifted in a large city. The following histograms show the distributions of the IQ scores of mothers and...
-
Shown below is activity for one of the products of Denver Office Equipment: January 1 balance, 700 units @ $55 per unit $38,500 Purchases: January 10: 700 units @ $60 per unit January 20: 1,100 units...
-
Malaysian Agrifood Corporation Berhad reported sales of RM 7 0 , 0 0 0 in May and RM 8 0 , 0 0 0 in June. The forecast sales for July, August and September are RM 9 0 , 0 0 0 , RM 1 0 0 , 0 0 0 , and...
-
1. Refer to the \"Plotting Data\" lesson (end of "Patterns to Notice") and plot the "Skydiver Velocity vs. Time\" data (taken from the video) on the following graph. to c) d) e) f) 9) Which is the...
-
How is machinability defined?
-
What can scientists learn by comparing the fossilized skeletons of extinct primates with the bones of modern species?
-
Indicate how the following items are recorded in the accounting records in the current year of Coronet Co. (a) Impairment of goodwill. (b) A change in depreciating plant assets from accelerated to...
-
Whittier Construction Co. had followed the practice of expensing all materials assigned to a construction job without recognizing any salvage inventory. On December 31, 2010, it was determined that...
-
Parsons Inc. wishes to change from the completed-contract to the percentage-of-completion method for financial reporting purposes. The auditor indicates that a change would be permitted only if it is...
-
Data Table The adjusted trial balance of Emes Real Estate Appraisal at June 30, 2024, follows: Click the icon to view the adjusted trial bsanen) Read the requirement Um Requirement 1. Prepare the...
-
Suppose you are the CFO of a large firm. Would you prefer to show company earnings based on the all-inclusive format or the current operating performance format, present GAAP notwithstanding? Explain...
-
Using an Aging Schedule to Account for Bad Debts Sparkle Jewels distributes fine stones. It sells on credit to retail jewelry stores and extends terms that require the stores to pay in 60 days. For...

Study smarter with the SolutionInn App


