To establish the law of conservation of mass, Lavoisier carefully studied the decomposition of mercury(II) oxide: (a)
Question:
To establish the law of conservation of mass, Lavoisier carefully studied the decomposition of mercury(II) oxide:
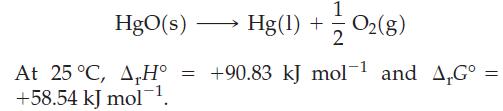
(a) Show that the partial pressure of O2(g) in equilibrium with HgO(s) and Hg(l) at 25 °C is extremely low.
(b) What conditions do you suppose Lavoisier used to obtain significant quantities of oxygen?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:





